
Acute respiratory failure 36 hours after a left lower lobectomy
Kondili Eumorfia, MDXirouchaki Nektaria
Georgopoulos Dimitrios, MD
Department of ICU , University Hospital of Heraklion , Crete Greece
Case Presentation
A 70-year-old man was admitted to the intensive care unit with acute hypoxemic respiratory failure. 48 hours earlier, he underwent a surgical resection of the lower lobe of the left lung for stage IIIB adenocarcinoma of the lung. During the 6-hour operation, he received a total fluid infusion of 5.5 L (including 3 units of packed red blood cells). The cumulative fluid infusion given during the peri-operative period (during surgery and the first 24 hours post-op) was 8.0 L with a net negative 0.7L. While the patient was in the recovery room, the endotracheal tube was removed without complications, and he transferred to the ward a few hours later. Approximately 36 hours later, dyspnea and hypoxemia were noted, and after 4 hours of continued hypoxemia, the trachea was inutbated to facilitate mechanical ventilation.
Past Medical History
Adenocarcinoma of the lung, stage IIIb, diagnosed 3 months before surgery, treated with preoperative neoadjuvant chemotherapy and radiotherapy. History of moderate-to-severe COPD.
Social History
80-pack-years of cigarette smoking; chronic alcohol consumption of approximately 70g of ethanol per day.
Pre-operative evaluation
Complete blood count and blood chemistry were normal.
Pre-operative evaluation for chronic heart disease was negative.
Forced Expiratory Volume in 1s (FEV1) was 1.79 L; 58% of the predicted value; calculated post-operative FEV1 was 49% of the predicted value.
ICU ADMISSION
Physical examination
Vital signs
BP 125/70 mmHg, Pulse 103/min, Respirations 18/min, Temperature 370 C
Cardiovascular
S1, S2 normal
Respiratory
Decreased breath sounds over the left lower lung field, diffuse end-inspiratory crackles over the remaining lobes.
Laboratory Data
- Normal complete blood count and chemistry
- Blood and bronchoalveolar lavage (BAL) specimens were collected and sent for microbiologic analysis. Two days later the results were negative for pathologic microorgamisms.
- Arterial blood gases: (on FiO2 0.6), PO2 70mmHg, PO2/ FiO2 117, PaCO2 45mmHg, HCO3 24, SaO2 92%
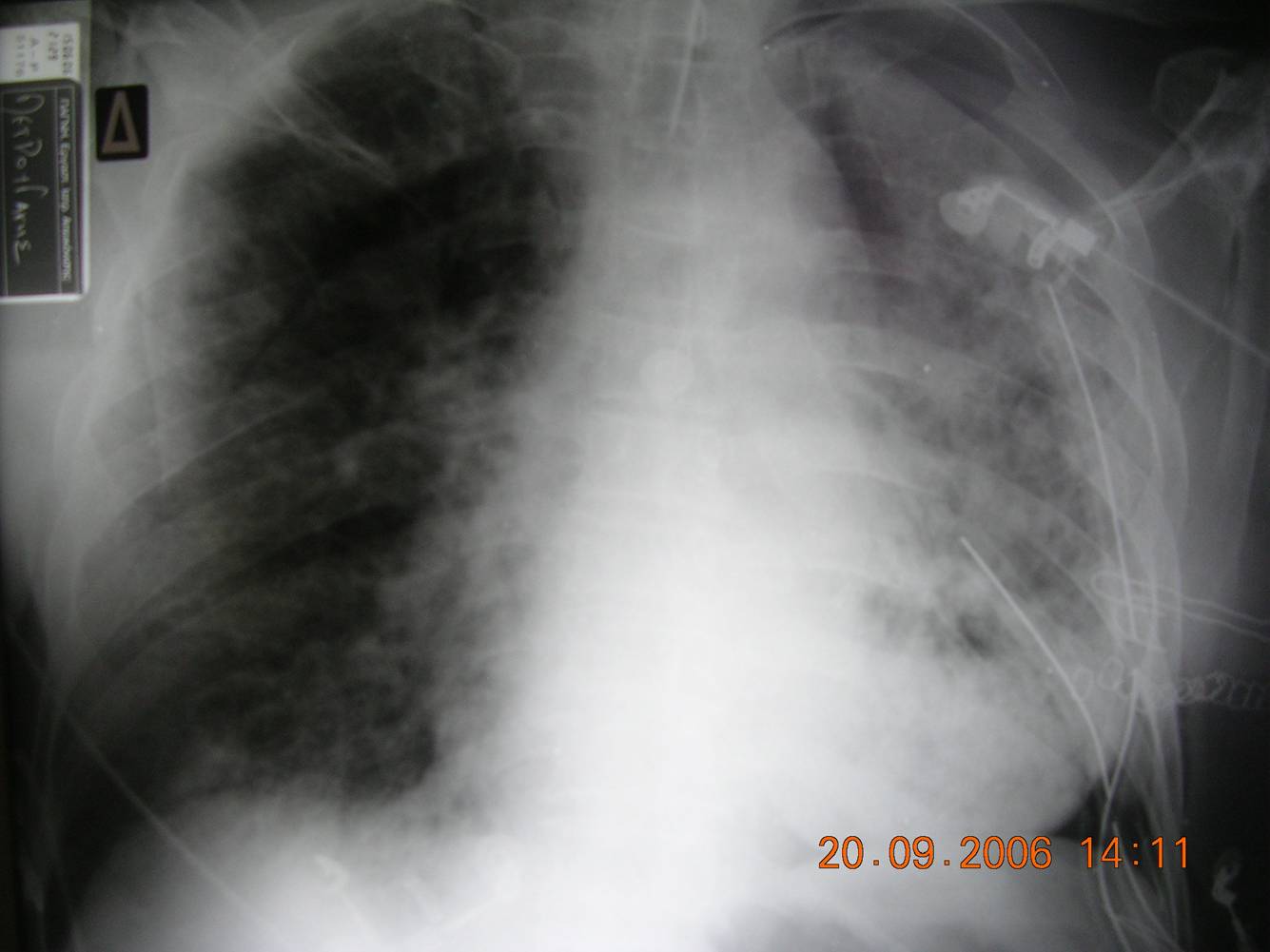
- Chest radiograph (Fig 1): Diffuse bilateral pulmonary infiltrates consistent with pulmonary edema.
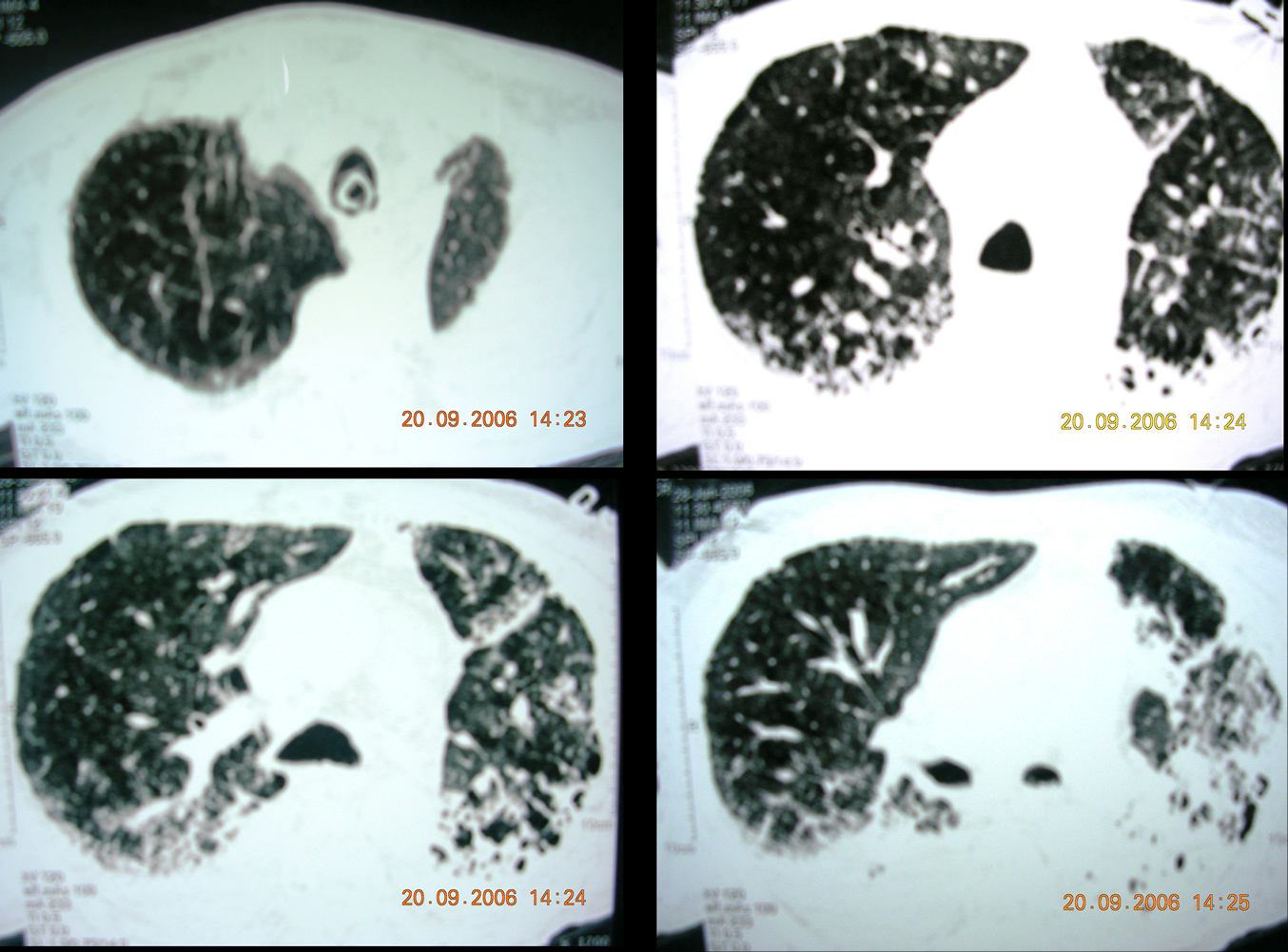
- Chest Spiral CT with PE protocol (Fig 2): patchy consolidations with increased interstitial markings and alveolar filling, no filling defects in the pulmonary arteries
- EKG: Sinus tachycardia
- Transthoracic echocardiography: Ejection fraction 60 %, normal left ventricular systolic function. Mild right ventricular dilation.
- Right heart catheterization:
Cardiac Output (CO): 7.74 L/min (normal 5-7 L/min)
Cardiac Index (CI): 4.8 L/min/m2(normal 3-5 L/min/m2)
CVP 8 mmHg
SVRI: 960 dynes/sec/cm5/m2 (normal 1200-1800)
Pulmonary artery systolic pressure (PASP): 59 mmHg
Pulmonary Wedge Pressure: 11 mmHg
ICU Course
On admission to the ICU, the patient was sedated and placed on volume control mechanical ventilation with the follow settings: FiO2: 0.6, VT: 450 ml, RR:18, PEEP:10 cm H2O, VΕ:8 L/min. Additional supportive therapy included initial, empiric, broad-spectrum antibiotics and restrictive fluid management.
On Day 3, due to further impairment of oxygenation (SaO2 <80%) that did not improve with increases in both PEEP and FiO2, the patient was placed on high frequency oscillatory ventilation. Although he had an initial improvement in oxygenation, his overall condition continued to decline and he died on Day 5 due to multiple organ failure.
Question 1
Which of the following statements is true: The development of acute respiratory failure in this patient was due to
A. Pulmonary edema due to fluid overload
B. Cardiogenic pulmonary edema due to left-sided heart failure
C. Acute lung injury/Acute respiratory distress syndrome (ALI/ARDS)
D. Pneumonia
E. Massive pulmonary embolism
Answer to Question 1
Correct answer: C.
Two key pieces of evidence argue against answer choice (A), pulmonary edema due to fluid overload:
- Net negative peri-operative fluid balance of -0.7 L
- Pulmonary artery wedge pressure of 11 mmHg
Likewise, several pieces of evidence argue against answer choice (B), Cardiogenic pulmonary edema due to left-sided heart failure:
- Pre-operative evaluation that did not reveal chronic heart disease
- Right heart catheterization that showed normal pulmonary artery wedge pressure and normal-to-high cardiac output
- Transthoracic echocardiogram, that showed normal left ventricular contractility and estimated ejection fraction
The results of the broncho-alveolar lavage (no pathologic micro-organisms) and the computed tomogram-pulmonary angiogram (patent pulmonary arteries) argue against the diagnoses of pneumonia, answer choice (D), and pulmonary embolism, answer choice (E) respectively.
The patient developed non-cardiogenic pulmonary edema that meets the criteria of Acute Lung Injury/Acute Respiratory Distress Syndrome (ALI/ARDS) established by the American-European Consensus Conference in 1994 1:
1. Sudden onset of hypoxemia (ALI: PaO2/FiO2 ≤300 mmHg; ARDS: PaO2/FiO2 ≤200 mmHg);
2. Diffuse bilateral pulmonary infiltrates on the chest radiograph, consistent with pulmonary edema;
3. No clinical evidence of left atrial hypertension or wedge pressure <18 mmHg .
Table 1 Consensus definition of TRALI
|
In this patient, ALI/ARDS might be attributed to various causes, such as infection, aspiration of gastric contents, and transfusion related acute lung injury (TRALI)1-3, however, clinical and laboratory findings were not indicative of any of these conditions.
In the absence of the other identifiable causes of ALI/ARDS, the likely cause is a clinical condition known as ALI/ARDS after lung resection.
Previously called post-pneumonectomy edema, ALI/ARDS after lung resection represents a form of non-cardiogenic pulmonary edema that develops after lung resection (usually pneumonectomy, lobectomy, or bilobectomy), in the absence of other identifiable causes6-8. This condition, which was first recognized as a distinct clinical entity by Zeldin in 19848, has similar clinical, radiological, and histopathological characteristics as ALI/ARDS and therefore might be considered one of its variants7. Most patients present between 1 and 3 days postoperatively, with refractory hypoxemia, disorientation, restlessness, dyspnea, tachypnea, and tachycardia.
Radiologic findings lag behind the clinical signs and may include a new interstitial pattern of edema in the early phase or diffuse parenchymal consolidation later in disease process. Histopathologically, it is characterized by diffuse alveolar damage.
The pathophysiology of ALI/ ARDS after lung resection consists of alteration in pulmonary vascular control and permeability. Pulmonary hypertension due to hypoxic vasoconstriction contributes to the development of alveolar edema by affecting hydrostatic forces. Hemodynamic shear stress injures capillary endothelium, allowing a protein-rich fluid to fill the interstitium and alveolar space. Impaired lymphatic drainage, due to preoperative radiotherapy, surgical dissection or tumor invasion, can further aggravate postoperative lung edema6,7.
Various theories have suggested factors that may contribute to the pathogenesis of this condition, including both surgical or perioperative factors and biochemical/inflammatory mediators6,7,9-13. A hypothetical model for the pathogenesis has been proposed by Jordan et al.7 According to this hypothesis, physical and biochemical alterations in both the ipsilateral and contralateral lung during one-lung ventilation, followed by ischemia-reperfusion injury, initiate the inflammatory process by release of reactive oxygen and nitrogen species. This then leads to cellular damage and the development of ALI and ARDS.
Question 2
Which of the following statements is true?
A. The incidenceof ALI/ARDS after lung resection is high (>10%) and not related to the type of resection.
B. The incidenceof ALI/ARDS after lung resection is low (<10%) and not related to the type of resection.
C. The mortality of ALI/ARDS after lung resection is low (<10%) and related to the type of resection.
D. The mortality of ALI/ARDS after lung resection is high (>10%) and related to the type of resection.
Answer to Question 2
Correct answer: D.
The overall incidence of ALI/ARDS after lung resection ranges from 2-4%11,14-19, but some investigators report an incidence as high as 12-15% with the inclusion of cases of mild edema10,20. In general the incidence is influenced by the type of operation, with a higher incidence after pneumonectomy (4-8%) than after lobectomy or bilobectomy and sub-lobar resections (1.0-7.5% and 0.81-3.2%, respectively)14-16,19-21. Kutlu et al.19 reported on 1139 patients and showed that the incidence of ALI/ARDS after lung resection was3.9 %. In that study, a higher incidence was found after extensive resection (12.9%), as compared with pneumonectomy (6%), lobectomy (3.7%), and minor resection (1%) 19. Ruffini et al. 16 reported an overall incidence of 2.2%. According to the type of resection the incidence was higher following right pneumonectomy (4.5%), followed by sublobar resection (3.2%), left pneumonectomy (3%), bilobectomy(2.4%), and lobectomy (2%) 16.
ALI/ARDS after lung resection has a high mortality, ranging from 40-72%, and the type of resection influences mortality14-16,19,21. In a study of 1121 patients who underwent pulmonary resection, Ruffini et al. reported an overall mortality of 2.8% and a disease specific mortality of 41% among patients who developed ALI/ARDS after lung resection16. According to the extent of the resection the mortality was higher following right pneumonectomy (57%) than extended operation and sublobar resections. In a retrospective study of 2192 lung resections, Dulu et al. found a mortality of 40%. The mortality was higher after pneumonectomy (50%), followed by lobectomy (42%) and sublobar resections (22%)15. In the study of Kutlu et al., ALI/ARDS after lung resection accounted for 72.5% of the total mortality after lung resection, with higher mortality observed following extensive resection (7.4%), followed by pneumonectomy (4.5%), lobectomy (2.2%), and minor resection (0.7%)19
Question 3
Which of the following factors have been identified as independent risk factors for ALI/ARDS after lung resection?
A. Excessive perioperative fluid administration
B. Alcohol abuse
C. Predicted postoperative lung function.
D. All of above
Answer to Question 3
Correct answer: D
In the current literature several studies have been aimed at identifying the risk factors for ALI/ARDS after lung resection.
Table 2. Risk factors for ALI/ARDS after lung resection
Author |
Year |
# subjects |
Significant Risk Factors Identified |
|
Alam et al. 14 |
2007 |
1428 |
|
|
Licker et al.21 |
2003 |
879 |
|
|
Ruffini et al.16 |
2001 |
1,221 |
|
|
Kutlu et al. 19 |
2000 |
1139 |
|
|
Parquin et al. 10 |
1996 |
146 |
|
|
van der Werff |
1997 |
197 |
|
Most published studies on the association between perioperative fluid administration and the development of the syndrome have yielded contradictory results. Several studies have demonstrated an association between the development ALI/ARDS after lung resection and fluid administration during the first 24 h after surgery in excess of 3-4 L;8,10,14,21however, this relationship has not been reported in centers with restrictive perioperative fluid management 22. In a retrospective study, Parquin et al. showed that an intraoperative fluid input > 2 L was an independent risk factor for ALI/ARDS10. Licker et al. reported on 879 patients who underwent pulmonary resection. The multivariate analysis in this study showed that excessive fluid administration (> 4 L) was an independent risk factor for the development of ALI/ARDS after lung resection (odds ratio 2.91, CI 1.87-7.38) 21. Similarly, in a recent study of 1428 patients, Alam et al. showed that patients with ALI/ARDS after lung resection received significantly higher median amounts of perioperative fluids than those in the control group (2775ml versus 2500 ml), with an odds ratio of 1.2 per increase of 500 ml in perioperative fluid administration 14.
Chronic alcohol abuse is associated with an increased incidence of ARDS and increased severity of multiple organ dysfunction in patients with septic shock and other diagnoses associated with the development of ARDS 3,23-25. Experimental data have shown that chronic ethanol abuse decreases pulmonary glutathione concentrations and subsequently impairs surfactant synthesis and secretion, increases the rate of apoptosis of type II cells, and alters alveolar-capillary permeability 23-25. All of these factors increase the risk of ALI/ARDS. Currently only one study has identified chronic alcohol abuse as an independent risk factor for ALI/ARDS after lung resection 9. In the study of 879 patients who underwent lung resection, Licker et al. observed a twofold increase in the incidence ALI/ARDS among patients with average ethanol consumption >60gr/day9.
The relation between post-operative predicted lung function and the development of ALI/ARDS after lung resection has been observed in two retrospective studies10,14. Parquin et al. studied 146 patients after pneumonectomy and demonstrated that a postoperative DLCO < 55% and FEV1 < 45 % were associated with ALI/ARDS after lung resection 10. Alam et al., in a recent series of 1428 patients, found that the patients who developed ALI/ARDS after lung resection had lower median postoperative predicted lung function values than those who not developed the syndrome14. This difference was reflected both by FEV1 (59% vs 69%) and DLCO (48% vs 60%); the odds ratios for developing ALI/ARDS were 1.11 and 1.10 for each 5% decrease in postoperative predicted FEV1 and DLCO, respectively14.
References
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R: The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149: 818-24
- Ware LB, Matthay MA: The acute respiratory distress syndrome. N Engl J Med 2000; 342: 1334-49
- Artigas A, Bernard GR, Carlet J, Dreyfuss D, Gattinoni L, Hudson L, Lamy M, Marini JJ, Matthay MA, Pinsky MR, Spragg R, Suter PM: The American-European Consensus Conference on ARDS, part 2. Ventilatory, pharmacologic, supportive therapy, study design strategies and issues related to recovery and remodeling. Intensive Care Med 1998; 24: 378-98
- Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, O'Byrne MM, Evenson LK, Malinchoc M, DeGoey SR, Afessa B, Hubmayr RD, Moore SB: Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med 2007; 176: 886-91
- Sachs UJ: Pathophysiology of TRALI: current concepts. Intensive Care Med 2007; 33 Suppl 1: S3-S11
- Gothard J: Lung injury after thoracic surgery and one-lung ventilation. Curr Opin Anaesthesiol 2006; 19: 5-10
- Jordan S, Mitchell JA, Quinlan GJ, Goldstraw P, Evans TW: The pathogenesis of lung injury following pulmonary resection. Eur Respir J 2000; 15: 790-9
- Zeldin RA, Normandin D, Landtwing D, Peters RM: Postpneumonectomy pulmonary edema. J Thorac Cardiovasc Surg 1984; 87: 359-65
- Licker M, Spiliopoulos A, Frey JG, Robert J, Hohn L, de Perrot M, Tschopp JM: Risk factors for early mortality and major complications following pneumonectomy for non-small cell carcinoma of the lung. Chest 2002; 121: 1890-7
- Parquin F, Marchal M, Mehiri S, Herve P, Lescot B: Post-pneumonectomy pulmonary edema: analysis and risk factors. Eur J Cardiothorac Surg 1996; 10: 929-32; discussion 933
- Patel RL, Townsend ER, Fountain SW: Elective pneumonectomy: factors associated with morbidity and operative mortality. Ann Thorac Surg 1992; 54: 84-8
- Williams EA, Quinlan GJ, Goldstraw P, Gothard JW, Evans TW: Postoperative lung injury and oxidative damage in patients undergoing pulmonary resection. Eur Respir J 1998; 11: 1028-34
- Williams EA, Quinlan GJ, Anning PB, Goldstraw P, Evans TW: Lung injury following pulmonary resection in the isolated, blood-perfused rat lung. Eur Respir J 1999; 14: 745-50
- Alam N, Park BJ, Wilton A, Seshan VE, Bains MS, Downey RJ, Flores RM, Rizk N, Rusch VW, Amar D: Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg 2007; 84: 1085-91; discussion 1091
- Dulu A, Pastores SM, Park B, Riedel E, Rusch V, Halpern NA: Prevalence and mortality of acute lung injury and ARDS after lung resection. Chest 2006; 130: 73-8
- Ruffini E, Parola A, Papalia E, Filosso PL, Mancuso M, Oliaro A, Actis-Dato G, Maggi G: Frequency and mortality of acute lung injury and acute respiratory distress syndrome after pulmonary resection for bronchogenic carcinoma. Eur J Cardiothorac Surg 2001; 20: 30-6, discussion 36-7
- Turnage WS, Lunn JJ: Postpneumonectomy pulmonary edema. A retrospective analysis of associated variables. Chest 1993; 103: 1646-50
- Waller DA, Gebitekin C, Saunders NR, Walker DR: Noncardiogenic pulmonary edema complicating lung resection. Ann Thorac Surg 1993; 55: 140-3
- Kutlu CA, Williams EA, Evans TW, Pastorino U, Goldstraw P: Acute lung injury and acute respiratory distress syndrome after pulmonary resection. Ann Thorac Surg 2000; 69: 376-80
- van der Werff YD, van der Houwen HK, Heijmans PJ, Duurkens VA, Leusink HA, van Heesewijk HP, de Boer A: Postpneumonectomy pulmonary edema. A retrospective analysis of incidence and possible risk factors. Chest 1997; 111: 1278-84
- Licker M, de Perrot M, Spiliopoulos A, Robert J, Diaper J, Chevalley C, Tschopp JM: Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 2003; 97: 1558-65
- Moller AM, Pedersen T, Svendsen PE, Engquist A: Perioperative risk factors in elective pneumonectomy: the impact of excess fluid balance. Eur J Anaesthesiol 2002; 19: 57-62
- Guidot DM, Roman J: Chronic ethanol ingestion increases susceptibility to acute lung injury: role of oxidative stress and tissue remodeling. Chest 2002; 122: 309S-314S
- Moss M, Burnham EL: Chronic alcohol abuse, acute respiratory distress syndrome, and multiple organ dysfunction. Crit Care Med 2003; 31: S207-12
- Moss M, Bucher B, Moore FA, Moore EE, Parsons PE: The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. Jama 1996; 275: 50-4



