Contributed by Gaurav Nigam MD1, Charu Pathak MBBS2, Muhammad Riaz MD, MS1 1Sleep Disorders Center, University of Michigan Health System, Ann Arbor, MI 2Government Medical College, Jabalpur, India Corresponding Author: Gaurav Nigam University of Michigan Sleep Disorders Center C728 Med Inn Bldg. | 1500 E. Medical Center Drive Ann Arbor, MI 48109-5845 Phone: 612.916.2993 | dr.nigamgaurav@gmail.com Disclosures: Authors do not have anything to disclose, this study was not supported by industry. The views expressed herein are solely of the authors. Conflict of Interest: None Keywords: treatment emergent central sleep apnea, central apnea, continuous positive airway pressure, central apnea index, apnea-hypopnea index, obstructive sleep apnea
In Brief
A middle age man with a normal body mass index presented for evaluation of snoring and excessive daytime sleepiness. Polysomnography revealed severe obstructive sleep apnea. A new pattern of sleep disordered breathing emerged during a CPAP titration study and became worse as titration progressed.
Case Vignette
A 64-year-old man with a past medical history of hypertension and atrial fibrillation presented with complaints of snoring and excessive daytime sleepiness ongoing for 5 years prior to presentation. He denied tobacco or recreational drug use. He consumed a glass of wine every weekend. His medications included simvastatin, losartan, metoprolol and a baby aspirin. The man’s body mass index (BMI) was 25 kg/m2.
He underwent an in-laboratory baseline polysomnogram that demonstrated severe obstructive sleep apnea (OSA) with an apnea hypopnea index (AHI) of 34, central apnea index (CAI) of 2 and an oxygen saturation nadir of 85%. Subsequently, the patient underwent an in- laboratory CPAP titration. Settings of 4-7 cm of water with C-flex of 3 cm were tested. The titration hypnogram is shown in Figure-1. Compared to the baseline study, the overall AHI dropped from 34 to 5.9. The changes from baseline in respiratory parameters on this study are summarized in Table 1. As noted in Figure-2, a new pattern of sleep disordered breathing emerged with initiation of CPAP therapy, which worsened as titration progressed (Figure-3). No Cheyne-Stokes breathing pattern was noted at any point during the titration study.
| Diagnostic Night | Diagnostic Night | ||
|---|---|---|---|
| Total Recording Time (TRT, min/hr) |
430.0/7:10.0 | Total Recording Time (TRT, min/hr) |
470.0/7:50.0 |
| Total Sleep Time (TST, min) |
315 | Total Sleep Time (TST, min) |
365.5 |
| Latency to sleep | 18 | Latency to | 7.5 |
| (min) | sleep (min) | ||
| REM Latency (min) | 220.0 | REM Latency (min) |
200.0 |
| Apnea Hypopnea Index |
34 | Apnea Hypopnea Index |
5.9 |
| Respiratory disturbance Index |
34 | Respiratory disturbance Index |
5.9 |
| Min % SpO2 | 85 | Min % SpO2 | 90 |
| Obstructive Apnea Index |
3.2 | Obstructive Apnea Index |
0.0 |
| Central Apnea Index | 2 | Central Apnea 5.1 |
Index |
| Hypopnea Index | 28.8 | Hypopnea 0.8 | Index |
| RERA Index | 0.0 | RERA 0.0 |
Index |
†Index = no./hour of sleep
Min: Minutes
Table 1: Sleep study report from diagnostic and titration nights with respiratory data analysis
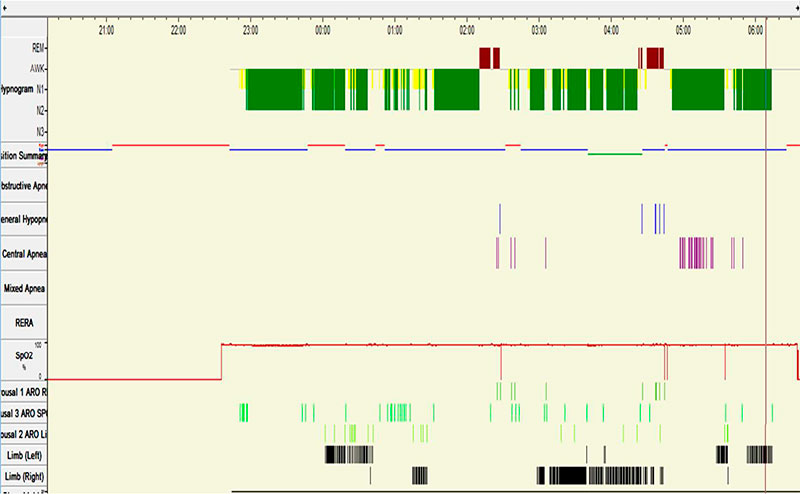
Figure 1: Titration Hypnogram depicting sleep stages, respiratory events and sleep position*
*Sleep position:
Blue line: Supine position
Red line: Right lateral position
Green line: Left lateral position
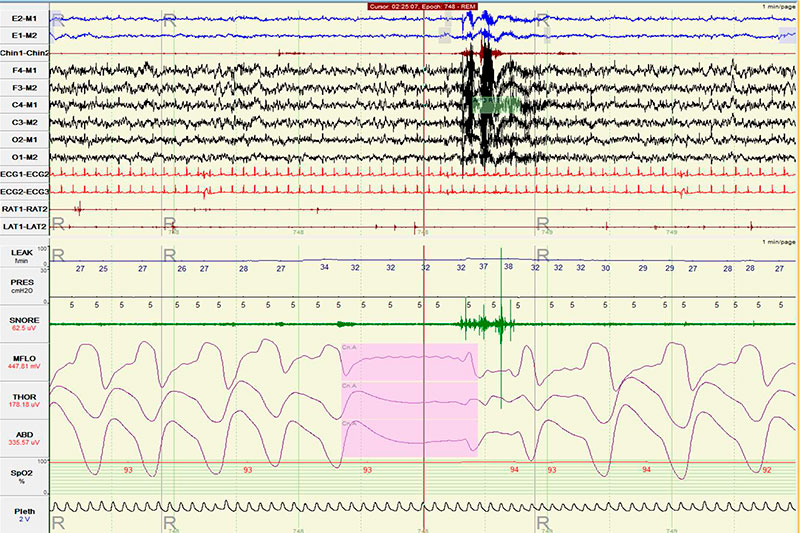
Figure 2: A new sleep disordered breathing pattern emerged as CPAP was initiated. One such 15 second-long event is shown on CPAP=5 cm. Notice the high mask leak at 32 liters/minute.
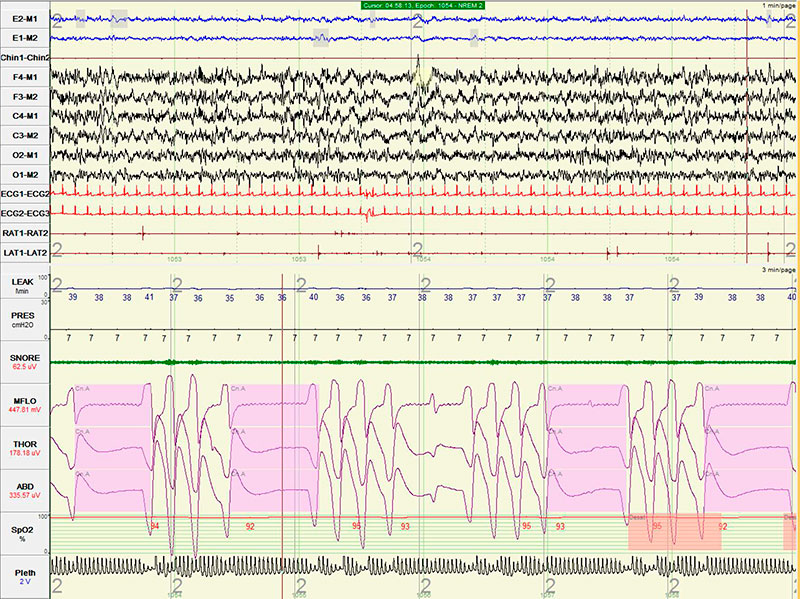
Figure 3: Worsening of the emerging sleep disordered breathing, with most frequent and longest lasting (18-22 seconds) events noted at CPAP of 7 cm (highest tested CPAP setting). Notice the high mask leaks up to 41 liters/minute.
Questions
-
Does the patient meet the polysomnographic criteria for treatment emergent central sleep apnea?
-
At what CPAP pressure settings do treatment emergent central sleep apneas typically appear?
-
What steps would you suggest to the sleep technician when he or she encounters central apnea noted as shown in Figure-2?
Discussion
Figures 1 and 2 document emergence of central sleep apnea as obstructive sleep apnea resolved in response to increasing levels of CPAP.
Treatment emergent central sleep apnea is the polysomnographic identification of central apneas and hypopneas after improvement or resolution of obstructive sleep apnea has been accomplished while undergoing positive airway pressure titration. The prevalence of treatment emergent central sleep apnea varies between 5-20 % (1, 2).
As outlined in the International Classification of Sleep disorders (ICSD-3) (3), all of the following criteria must be met for a diagnosis of emergent central sleep apnea during a PAP titration study (CPAP or bi-level PAP without a back up rate): There should be significant resolution of obstructive events with PAP therapy which inadvertently leads to development of all of these 3 conditions:
-
Central apnea—central hypopnea index [CAHI] ≥ 5/hour.
-
Number of central apneas and central hypopneas is ≥ 50% of total number of apneas and hypopneas.
-
The central sleep apnea is not better explained by another CSA disorder (e.g., CSA with CSB or CSA due to a medication or substance).
Treatment-emergent central sleep apnea has also been described after treatment of OSA using non-PAP treatment modalities such as use of a mandibular advancement device (4), tracheostomy (5), maxilla-mandibular advancement (6), and nasal surgery (7).
Most studies performed on patients with treatment-emergent central sleep apnea did not find one particular numerical high pressure setting that correlated best with emergence of central apnea. This phenomenon can occur at any tested CPAP setting from 4 cm of water to 20 cm of water. However 2 studies (2, 8) did find that patients who develop treatment emergent central sleep apnea tended to require higher CPAP settings to abolish their obstructive apneas compared to controls that did not develop treatment emergent central sleep apnea.
Appearance of central apnea is related to a patient’s “apneic threshold”. The hypocapnia-induced apneic threshold is a physiological state-dependent, non-constant numerical value resulting from a drop in end tidal PaCO2 (usually 3-4 mm Hg) below the eupneic PaCO2. Higher pressures lead to pressure intolerance and mask leak, contributing to sleep fragmentation. This could result in drop of PaCO2 to a level below the apneic threshold, thereby triggering central apneas. Furthermore, in patients with OSA the withdrawal of “wakefulness drive” precipitates decreased and fluctuating oscillatory muscle tone in the upper airway leading to ventilator instability and central apneas (9).
Watchful waiting will lead to resolution of most cases of treatment-emergent central sleep apnea. If these central apneas become more frequent or protracted, or the patient develops significant arterial oxygen desaturation, one can try lowering the CPAP pressure. This will mitigate pressure intolerance, mask leak, sleep fragmentation and hopefully promote sleep consolidation. Rapid titration with concurrent increased mask leaks could further increase the incidence of central apnea (10), as was noted in this patient. Hence a close attention to mask fitting is of utmost importance.
In some cases, positional therapy (avoidance of supine sleep) can be beneficial (11). If the patient has significant oxygen desaturation, one can try a small amount of supplemental oxygen. If treatment emergent central sleep apnea is necessitating a re-titration, consideration
can be given to use of bi-level PAP with spontaneous-timed (ST) mode (12).
At the same time, other causes of CSA should be investigated. In this patient, given lack of prior echocardiogram, we recommended evaluation for underlying heart disease.
Answers
-
Central sleep apnea with Cheyne-Stokes breathing.
-
Cheyne Stokes breathing is commonly associated with cardiac disease (i.e. heart failure). Other associations include neurological disease, renal failure, sedation, normal sleep, acid-base disturbances, prematurity, and altitude acclimatization.
-
Cheyne Stokes respiration can be treated with continuous positive airway pressure (CPAP), adaptive servoventilation (ASV), and nocturnal oxygen.
Follow-Up
Our patient was started on adaptive servoventilation and advised to follow up in Sleep Clinic after discharge from the hospital.
On return 1 month later, cardiovascular evaluation revealed a improvement in left ventricular ejection fraction to 25-30%. He did not return subsequently to our medical center after transfer of care other practices because of limitations in health insurance coverage.
If the patient’s left ventricular ejection fraction does not recover to greater than 45%, an alternative adaptive servoventilation should be explored for the reasons cited in the discussion. Alternative treatment options include supplemental oxygen, CPAP or CPAP with oxygen, although the long term effects of these modalities is unknown. Additionally, optimal treatment of underlying heart failure should continue to be a priority (18).
References
-
American Academy of Sleep Medicine. International classification of sleep disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine, 2014.
-
Berry B, Brooks R, Gamaldo C, Harding S, Lloyd R, Marcus C, Vaughn B. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Version 2.1
-
MacDonald M, Fang J, Pittman SD, et al. The current prevalence of sleep disordered breathing in congestive heart failure patients treated with beta-blockers. J Clin Sleep Med 2008;4:38–42.
-
Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. Jan 1996;153(1):272-6.
-
Luo Q, Zhang HL, Tao XC, Zhao ZH, Yang YJ, Liu ZH. Impact of untreated sleep apnea on prognosis of patients with congestive heart failure. Int J Cardiol. Apr 2 2009
-
Khayat R, Small R, Rathman L, et al. Sleep disordered breathing in heart failure: identifying and treating an important but often unrecognized comorbidity in heart failure patients. J Cardiac Fail 2013;19:431–44.
-
Caruana-Montaldo B, Fleeson K, Zwillich CW. The control of breathing in clinical practice. Chest 2000; 117:205-25
-
Khayat R, Abraham W, Patt B, Brinkman V, Wannemacher J, Porter K, Jarjoura D. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J Card Fail 2012;18: 534e540.
-
Ohmura T, Iwama Y, Kasai T, Kato T, Suda S, Takagi A, Hiroyuki D. Impact of Predischarge Nocturnal Pulse Oximetry (Sleep-Disordered Breathing) on Postdischarge Clinical Outcomes in Hospitalized Patients With Left Ventricular Systolic Dysfunction After Acute Decompensated Heart Failure. The American Journal of Cardiology 2014; 113:697-700. doi:10.1016/j.amjcard.2013.10.048
-
Aurora RN, Chowdhuri S, Ramar K, Bista SR, Casey KR, Lamm Cl, Kristo DA, Mallea JM, Rowley JA, Zak RS, Tracy SL. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. SLEEP 2012;35(1):17-40.
-
Arzt M, Floras J, Logan A, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian continuous positive airway pressure of patients with central sleep apnea and heart failure trial (CANPAP). Circulation 2007;115:3173-80.
-
Philippe C, Stoïca‐Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne‐Stokes respiration in heart failure over a six month period. Heart 2006; 92(3):337-. doi:10.1136/hrt.2005.060038.
-
Kasai T, Usui Y, Yoshioka T, et al. Effect of flow-triggered adaptive servoventilation compared with continuous positive airway pressure in patients with chronic heart failure with coexisting obstructive sleep apnea and Cheyne-Stokes respiration. Circ Heart Fail 2010;3:140-8
-
Sharma BK, Bakker JP, McSharry DG, Desai AS, Javaheri S, Malhotra A. Adaptive servoventilation for treatment of sleep-disordered breathing in heart failure Chest. 2012;142(5):1211-1221. doi:10.1378/chest.12-0815
-
Cowie MR, Woehrle H, Wegscheider K, Angermann C, d'Ortho MP, Erdmann E, Levy P, Simonds A, Somers VK, Zannad F, Teschler H. Rationale and design of the SERVE-HF study: treatment of sleep-disordered breathing with predominant central sleep apnoea with adaptive servo-ventilation in patients with chronic heart failure. Eur J Heart Fail. 2013 Aug;15(8):937-43. doi: 10.1093/eurjhf/hft051.
-
ResMed Press Release. Available at: http://www.resmed.com/us/en/consumer/newsandinformation/news-releases/2015/resmed-provides-update-on-phase-iv-serve-hf-study-of-adaptive-servo-ventilation-therapy.html. Accessed June 17, 2015.
-
American Academy of Sleep Medicine Special Safety Notice. Available at: http://www.aasmnet.org/articles.aspx?id=5562. Accessed June 17, 2015.
-
Ayas NT, Patil SP, Stanchine M, Malhotra A. Treatment of Central Sleep Apnea with Adaptive Servoventilation in Chronic Heart Failure. AM J Respir Crit Care Med. First published online 17 Jun 2015.



