Contributed by Omar A. Mesarwi1, Jeffrey M. Ellenbogen2, and Vsevolod Y. Polotsky1 1Division of Pulmonary and Critical Care Medicine, Department of Medicine, and 2Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, Maryland
In Brief
A 41-year-old patient with severe obstructivesleep apnea underwent a continuous positiveairway pressure titration study. The patientexhibited unusual findingsin rapid eye movement (REM) sleep.
A 41-year-old man presented complaining of loud, disruptive snoring. The snoring had been present for at least 8 years, corresponding with a 40-pound weight gain. He had never been told of witnessed apnea or of unusual movement during sleep. He worked as a manager of an apartment building complex with several thousand units and routinely worked 12 to 16 hours per day. He was otherwise well except for chronic depression, for which he took sertraline. He denied alcohol use but consumed two cups of caffeinated coffee in the morning and 5 to 10 servings of caffeine- containing carbonated beverages throughout the day. Physical examination revealed an obese man with body mass index of 36 kg/m2. Oropharyngeal examination revealed a Mallampati grade IV airway with small tonsils. Cardiac and pulmonary exams were unremarkable. A full neurologic exam was not performed, but the patient demonstrated grossly normal speech, gait, and motor function.
An overnight, in-lab polysomnogram was performed, which revealed severe obstructive sleep apnea, with a combined apnea-hypopnea index of 44.1 events/h, all of which were obstructive (9.7/h apneas, 34.5/h hypopneas). Sleep efficiency was 58%. In supine sleep (37% of total sleep time), there were 60.7 disordered breathing events/h, and in nonsupine sleep, there were 34.4. All stages of sleep were noted, and REM sleep comprised 22% of the total. No unusual events were noted during the sleep study.
The patient returned for an overnight continuous positive airway pressure titration study. During this study, two brief periods of abnormal EEG activity were recorded during REM sleep (Figure 1; Video 1). REM sleep comprised 25% of total sleep time, and sleep efficiency was 86%.
Questions
- What is the presumptive diagnosis, basedon the polysomnogram?
- What is the best next step for evaluation ofthis patient?
Discussion
Video monitoring revealed a brief episode of jerking head movements during REM sleep. There were no coincident abnormal movements of the left arm and no leg movements per the polysomnogram tracings. Clinically the patient had a nocturnal focal motor seizure, which occurred during REM sleep. His EEG during the event suggested the possibility of seizure activity (Figure 1A), but the discharge pattern was not clearly epileptiform (Figure 1B).
Figure 1. Polysomnogram tracings from the patient’s overnight continuous positive airway pressure titration study. (A) A 70-second epoch from the study. The patient is in REM sleep as evidenced by rapid eye movements in the first few seconds of the tracing. Abnormal eye movements, accompanied by abnormal chin electromyography and abnormal EEG tracings, are seen toward the end of the epoch. (B) A 10-second epoch shows abnormal EEG tracings more closely.
When video playback was slowed, we could see that the frequency of the EEG abnormality was similar to the frequency of the patient’s head movements. These observations suggest that the EEG did not record electrical seizure activity but instead captured motion artifact from a seizure. This is consistent with the fact that the electrodes used in this sleep study were placed only over the right hemisphere (F4, C4, O2), whereas the presumptive epileptic focus was in the left hemisphere (Figure 2).
Few studies have examined the relationship between sleep stage and the frequency of seizure activity, but those studies seem to indicate that seizure is uncommon in REM. Bazil and Walczak (1) analyzed over 1,100 seizures in 188 patients by examining video-EEG data for a mean patient monitoring duration of 6.4 days. Twenty percent of all seizures occurred during sleep. Complex partial seizure was by far the most common seizure subtype during sleep, comprising 85% of sleep- related seizures. Of the complex partial seizures during sleep, only 3% occurred during REM (1). A more recent study of 117 seizures in 55 patients who underwent either video-EEG polysomnography in a sleep lab or continuous video-EEG in an epilepsy unit corroborated these results, finding that 5% of observed seizures during sleep were in REM, with a relative frequency of 0.35 seizure events per hour in non-REM versus 0.09 seizures per hour in REM sleep (2). Another study of 613 seizures in 133 patients with refractory epilepsy found no seizures during REM sleep (3). Thus, REM sleep seizures are rare. The precise reason for this is unknown, although, unlike non-REM sleep, REM sleep is not characterized by synchronous activity between brainstem, thalamus, and cortex; this lack of synchronicity during REM may hinder seizure development (2, 4).
Determining the cause of a seizure in an adult patient can be difficult, and many adults presenting with their first seizure do not have an identifiable cause (5, 6). However, a reasonable initial evaluation of a patient with a seizure should include neuroimaging to exclude a structural brain abnormality. One cross-sectional study of adults with unprovoked seizures suggested that a substantial number of these patients will have ischemia, hemorrhage, trauma, or tumor as an underlying cause (6). These are all diagnoses that may be identified by computed tomography or magnetic resonance imaging, the latter generally being more sensitive.
All adults with an initial unprovoked seizure should have basic laboratory values measured, including renal and hepatic panels, thyroid function tests, electrolytes, glucose, hematology values, and possibly toxicology screens. Most
patients should undergo a dedicated EEG to discern the seizure subtype and to refine the prognosis. Only about 23% of patients have an epileptiform abnormality on EEG after an initial seizure (7), but this finding substantially increases the likelihood that a patient will have a second seizure within the next 2 years (8, 9). In selected patients in whom there is a suspicion for infection or meningeal spread of cancer, a lumbar puncture may also be performed.
Our patient had normal laboratory values. A dedicated EEG done after the continuous positive airway pressure titration study was also normal. MRI revealed the presence of a prominent left frontal lobe mass with a large cystic component and peripheral nodular and patchy enhancement, suggesting a high-grade glial tumor (Figure 2). Although the overall incidence of seizure decreases in middle age, the proportion due to tumor increases in this age group (10).
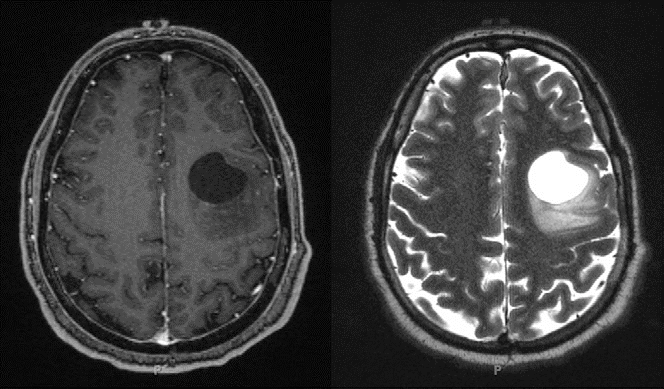
Figure 2. Brain MRI obtained shortly after the patient’s continuous positive airway pressure titration study. The patient was found to have a circumscribed cystic mass in the left middle frontal gyrus. Left panel: T1-weighted image postcontrast. Right panel: T2-weighted image.
Answers
- What is the presumptive diagnosis, basedon the polysomnogram?
The patient demonstrated rhythmic jerking movements of the head during REM sleep, consistent with a clinical diagnosis of focal motor seizure.
- What is the best next step for evaluation ofthis patient?
The evaluation of a first episode of unprovoked seizure in an adult should include laboratory assessment (electrolytes, glucose, toxicology, and renal/hepatic function panels), dedicated EEG, and neuroimaging.
Follow-Up
The patient underwent surgical resection of the mass. The lesion was identified as a gemistocytic astrocytoma with a small focus of anaplastic transformation. He completed chemoradiation with low dose temozolomide, followed by six cycles of adjuvant temozolomide, and has tolerated therapy well.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
-
Bazil CW, Walczak TS. Effects of sleep and sleep stage on epileptic and nonepileptic seizures. Epilepsia 1997;38:56–62.
-
Minecan D, Natarajan A, Marzec M, Malow B. Relationship of epileptic seizures to sleep stage and sleep depth. Sleep 2002;25:899–904.
-
Herman ST, Walczak TS, Bazil CW. Distribution of partial seizures during the sleep–wake cycle: differences by seizure onset site. Neurology 2001;56:1453–1459.
-
de Andre´ s I, Garzo´ n M, Reinoso-Sua´ rez F. Functional anatomy of non- REM sleep. Front Neurol 2011;2:70.
-
Schachter SC. Iatrogenic seizures. Neurol Clin 1998;16:157–170.
-
Forsgren L, Bucht G, Eriksson S, Bergmark L. Incidence and clinical characterization of unprovoked seizures in adults: a prospective population-based study. Epilepsia 1996;37:224–229.
-
Krumholz A, Wiebe S, Gronseth G, Shinnar S, Levisohn P, Ting T, Hopp J, Shafer P, Morris H, Seiden L, etal.; Quality Standards Subcommittee of the American Academy of Neurology; American Epilepsy Society. Practice parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology2007;69:1996–2007.
-
van Donselaar CA, Schimsheimer RJ, Geerts AT, Declerck AC. Value of the electroencephalogram in adult patients with untreated idiopathic first seizures. Arch Neurol 1992;49:231–237.
-
Das CP, Sawhney IM, Lal V, Prabhakar S. Risk of recurrence of seizures following single unprovoked idiopathic seizure. Neurol India 2000;48: 357–360.
-
Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia 1993;34:453–468.



