Not a Good ‘Short-Cut’ to Take!
Kunal Jakharia, MD, Pulmonary and Critical Care Fellow, University of North Carolina, Chapel Hill
Christine L. Vigeland, MD Clinical Instructor, University of North Carolina, Chapel Hill
Case:
55-year-old man with history of obesity, alcohol use disorder, untreated hepatitis C, with no prior history of lung disease, presented to the emergency room with shortness of breath. He was noted to be awake and alert with 1+ bilateral lower extremity edema. Oxygen hemoglobin saturation (O2 sat) was 85% on room air. An arterial blood gas on CPAP 12cm H20, FiO2 50% showed a PaO2 of 90 mmHg. Respiratory viral panel with COVID-19 PCR was negative, with a normal appearing chest X-ray. His platelet count and albumin were 125 x 103 /ul and 2.8 gm/dl respectively. He was admitted to the ICU for hypoxemia requiring high flow nasal cannula (HFNC) and intermittent CPAP. He was noted to have a decrease in his O2 sat while sitting upright, requiring up to 100% HFNC. As part of workup of his hypoxemia, he had a transthoracic echocardiogram performed that was a technically difficult study but showed a normal left ventricular ejection fraction, trace tricuspid regurgitation, and was unable to estimate right ventricular systolic pressure (RVSP). A perfusion scan was performed, which is shown below.
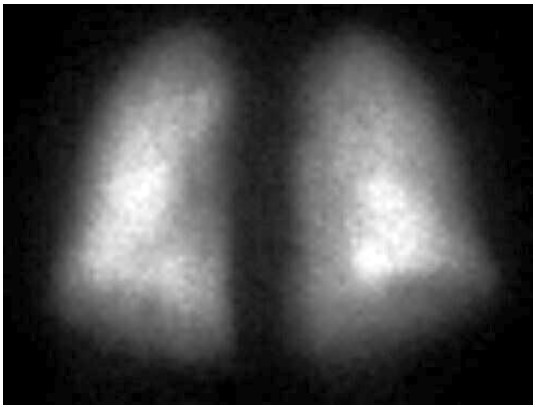
FIGURE A – Anterior
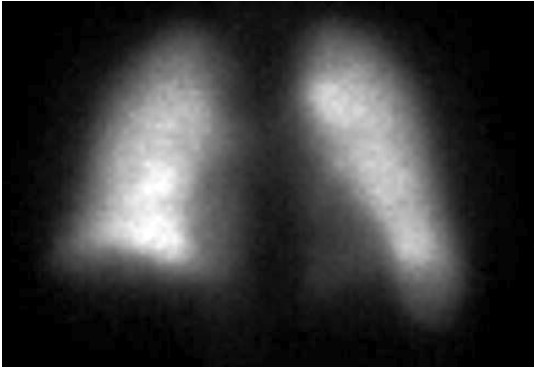
FIGURE B - Posterior
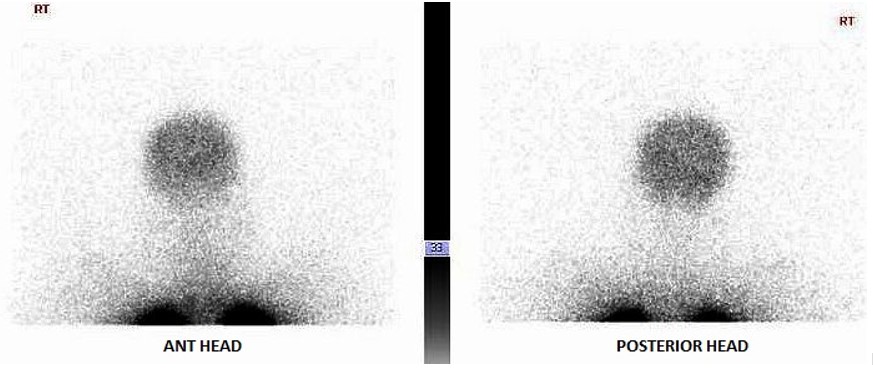
FIGURE C
Question:
What is the most likely cause of his hypoxemia?
- High probability pulmonary embolism (PE)
- Hepatopulmonary syndrome (HPS).
- Lobar pneumonia
- Porto pulmonary hypertension (PPH)
B. Hepatopulmonary syndrome
Discussion:
The perfusion scan demonstrates radiotracer uptake within the brain suggestive of a right to left shunt. With the history of alcohol use disorder, untreated hepatitis C, thrombocytopenia and low albumin, the patient was suspected to have cirrhosis and/or portal hypertension, and a right upper quadrant ultrasound confirmed this. Liver disease, high A-a gradient (216 mmHg) on oxygen (FiO2 60%), with orthodeoxia raised suspicion for hepatopulmonary syndrome. A positive perfusion scan was followed by an echocardiogram with bubble study, which confirmed delayed presence of intravenous saline contrast in the left heart characteristic of an intrapulmonary shunt. A CT angiogram was then performed, which showed 2 small left lower lobe pulmonary arteriovenous malformations (AVMs), however, they were not amenable to any interventions.
Probability of PE was low as there was no evidence of a perfusion defect. The patient did not have any symptoms and signs suggestive of an active infection and there was no radiographic evidence of a lobar pneumonia. PPH can also cause hypoxia in patients with cirrhosis, but in that case the perfusion scan would not show evidence of a right to left shunt unless there was a concomitant intracardiac shunt. PPH would be suggested by signs of pulmonary hypertension on echocardiogram, such as dilated right ventricle and elevated RVSP, and confirmed with right heart catheterization. Although initial echocardiogram was technically limited which could have missed signs of pulmonary hypertension, PPH would not explain his orthodeoxia.
Hepatopulmonary syndrome (HPS)
Definition and Classification:
It is characterized by the presence of the following triad1:
- Arterial oxygenation defect (A-a gradient > 15 mmHg on room air)
- Intrapulmonary vascular dilatations (IPVD) as evidenced by Contrast enhanced echocardiography (CE-TTE) or abnormal uptake of radiotracer on lung perfusion imaging
- Liver disease (not necessarily with portal hypertension or cirrhosis as it has been reported in other conditions as well)
HPS occurs in 10-30% of patients with cirrhosis. The severity of HPS is determined by the degree of hypoxemia, graded from mild (Pa02 > 80 mmHg), moderate (Pa02 > 60 to < 80 mmHg), severe (Pa02 > 50 to < 60 mmHg) and very severe (Pa02 < 50 mmHg) by the ERS Task Force1.
Mechanisms of Hypoxemia and Pathophysiology in HPS:
Berthelot described the pathological findings of HPS in 1966 as widespread dilatations of pulmonary microvessels encompassing the pulmonary precapillary and alveolar capillary beds2. Impaired gas exchange occurs by 3 physiological mechanisms due to the IPVD:
- Diffusion limitation (most common) - The distance at the gas exchange interface increases due to the dilated microvessels. This is exacerbated by the rapid blood flow due to hyperdynamic circulation in some patients.
- V/Q mismatch – Increased perfusion of the less ventilated dependent lower zones with a blunted vasoconstriction response to hypoxemia.
- Shunt – Arteriovenous shunts are formed which bypass alveoli, increasing the mixed venous blood entering the pulmonary veins.
These IPVD and shunts occur primarily at lung bases, worsening the hypoxemia in sitting or standing position, as gravity forces more blood to flow through them. Orthodeoxia occurs when the patient sits up, as pulmonary blood flow is unevenly distributed worsening oxygenation. Supplemental oxygen should theoretically correct hypoxemia from causes a and b, but not c, which forms the basis of the shunt fraction test at 100% FiO2.
IPVD occur due to endogenous vasoactive molecules, like NO and ET-13, and pulmonary angiogenesis is thought to be a result of monocyte stimulation (causing increased TNF) from bacterial translocation which increases in patients with liver disease.
Key Investigations:
Hypoxemia in liver disease which does not improve with oxygen supplementation suggests shunt physiology and should be investigated with a CE-TTE once other causes of an elevated A-a gradient are ruled out. CT-TTE would pick up an intra-cardiac shunt if the bubbles of agitated saline are seen within 2-3 cardiac cycles on the left side or an intrapulmonary shunt if the bubbles are seen on the left side after 5-6 cardiac cycles. In a Tc-99m macroaggregated albumin (MAA) perfusion scan of the lung, radiotracer should be trapped in the pulmonary alveolar capillary bed. Thus, detection of radiotracer in the brain or kidneys suggests IPVD or anatomical shunts. MAA perfusion scan is highly specific but less sensitive than CE-TTE for detecting intrapulmonary shunting consistent with HPS. Therefore, MAA perfusion scan is a better test to perform in patients with coexistent lung disease due to its specificity3, 5.
Treatment
Liver transplant is currently the only effective treatment for HPS. Pentoxifylline (due to the role of TNF), methylene blue (inhibition of NO) and garlic have been studied, however no medical therapeutics have yet been established3.
Our patient was not a candidate for liver transplant and was discharged home on oxygen.
References
-
Rodriguez‐Roisin R, Krowka MJ, Herve P, Fallon MB. Pulmonary‐Hepatic vascular Disorders (PHD). Eur. Respir. J.2004; 24: 861–880
-
Berthelot P, Walker JG, Sherlock S, Reid L. Arterial changes in the lungs in cirrhosis of the liver—lung spider nevi. N. Engl. J. Med.1966; 274: 291–298.
-
Grace JA, Angus PW. Hepatopulmonary syndrome: update on recent advances in pathophysiology, investigation, and treatment. J Gastroenterol Hepatol. 2013;28(2):213-219. doi:10.1111/jgh.12061
-
Abrams GA, Jaffe CC, Hoffer PB, et al. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology. 1995;109:1283–1288.
-
El-Shabrawi MH, Omran S, Wageeh S. 99mTechnetium-macroaggregated albumin perfusion lung scan versus contrast enhanced echocardiography in the diagnosis of the hepatopulmonary syndrome in children with chronic liver disease. Eur J Gastroenterol Hepatol. 2010;22:1006–1012.



