An Elderly Woman with Acute Onset Chest Pain
John L. Temple MD, Internal Medicine Resident
Daniel A. Sweeney MD, Pulmonary Critical Care Attending
Michael J. Wilkinson MD, Cardiology Fellow
Lori B. Daniels MD, MAS Cardiology Attending
University of California - San Diego
San Diego, California
History of Present Illness: 77-year-old woman presented to the ED with two hours of acute onset of severe, sharp chest pain radiating to her right back.
Past Medical History: Atrial fibrillation on Warfarin, diastolic heart failure and hypertension.
Vital Signs: BP 106/33 mmHg; pulse 64/minute; respirations 15/minute; pulse oximetry 98% on 2L nasal cannula; temperature 98.2°F.
Physical Exam: Moderate distress, diaphoretic, alert and oriented, 3/6 systolic murmur best heard at right upper sternal border, 2/6 early diastolic murmur, JVP 8 cm H2O, radial pulses 2+ bilaterally.
ECG: Sinus bradycardia; T-wave inversions in leads II, III, aVF; 1mm down-sloping ST depressions in leads V4-V5.
Initial Labs: Creatinine 1.0 mg/dL; Hemoglobin 12.3 gm/dL; INR 2.4; Troponin-T <0.01 ng/mL.
Imaging: Chest X Ray revealed cardiomegaly without evidence of mediastinal widening, pulmonary edema, or pleural effusions.
Cardiac point-of-care ultrasound (POCUS) was performed and a representative video was obtained.
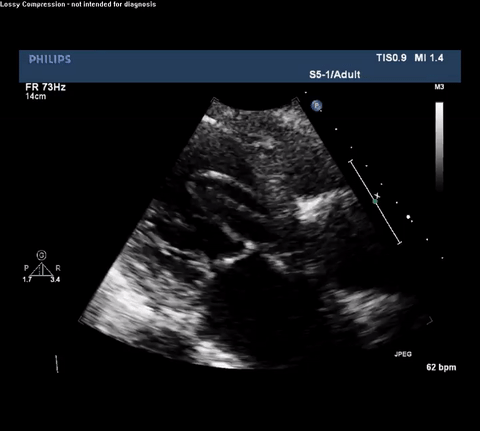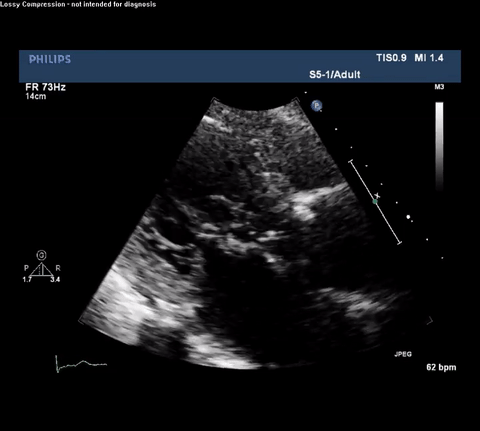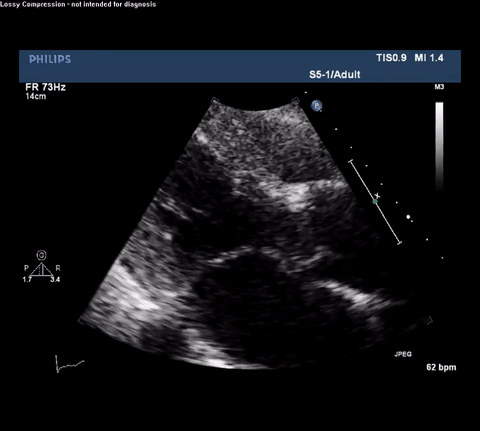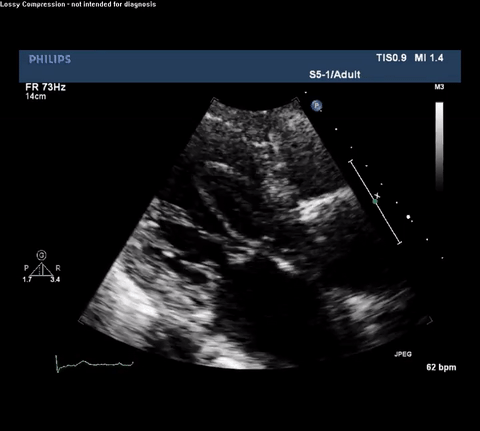
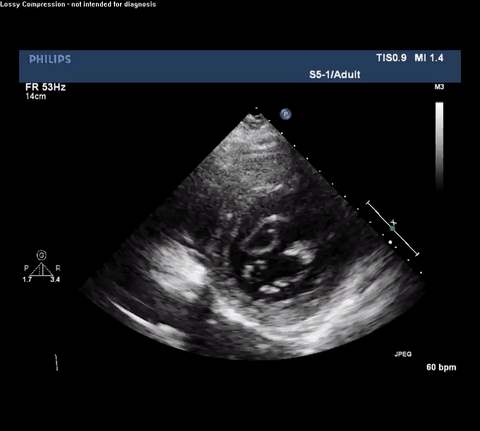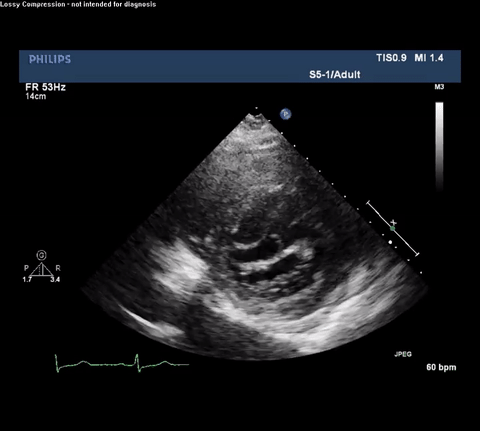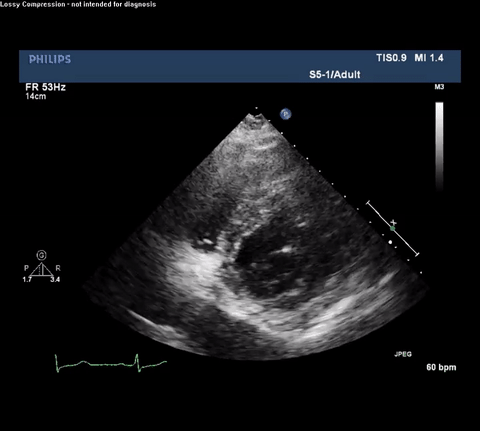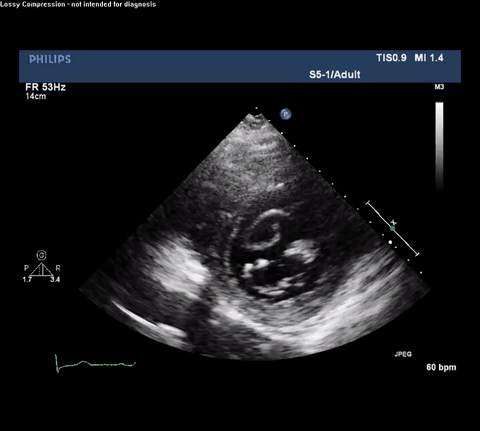
TTE Parasternal Long Axis View
Question:
What is the cause of this patient’s chest pain?
Type A acute aortic dissection (TA-AAD). Cardiac POCUS imaging in the parasternal long axis revealed the intima of the ascending aorta undulating in and out of the left ventricular outflow tract throughout the cardiac cycle, consistent with a TA-AAD.
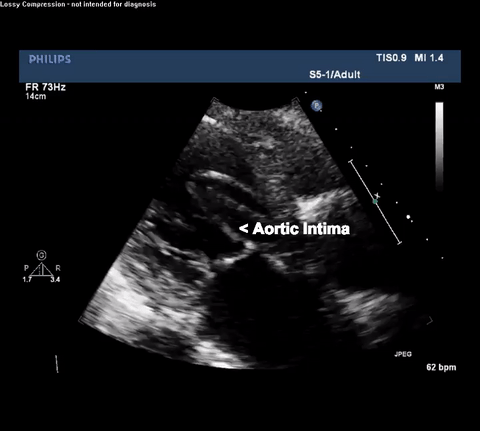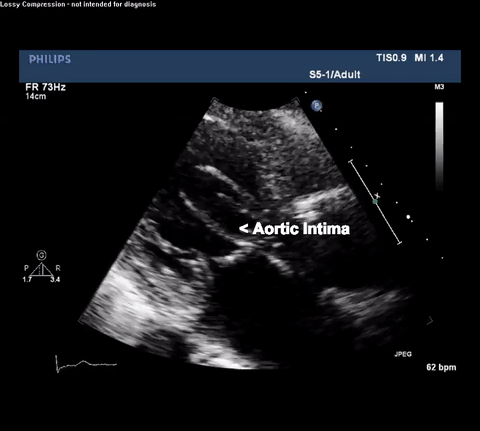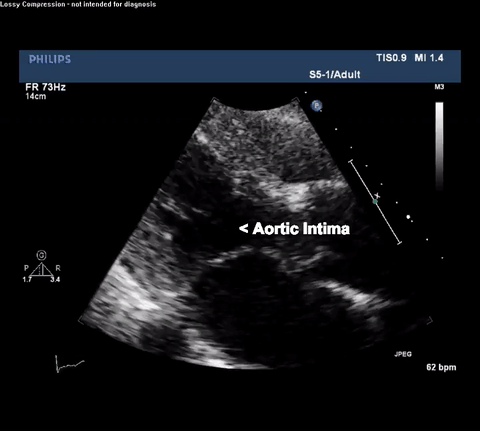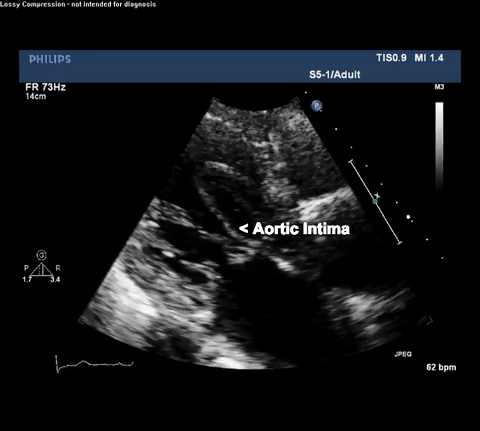
TTE Parasternal Long Axis View
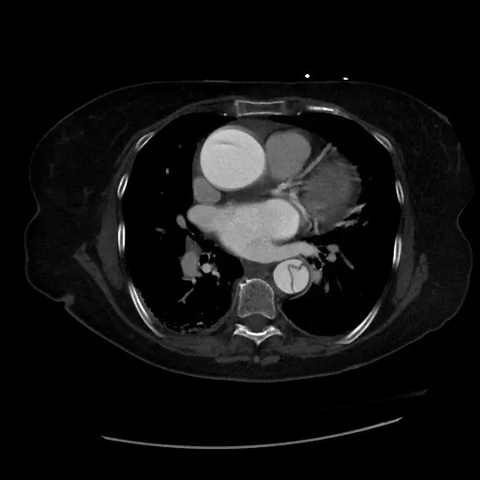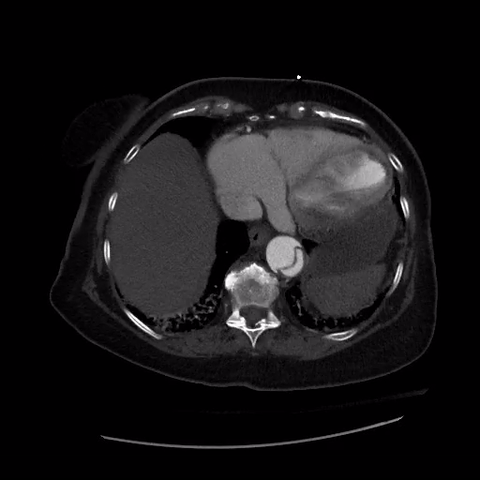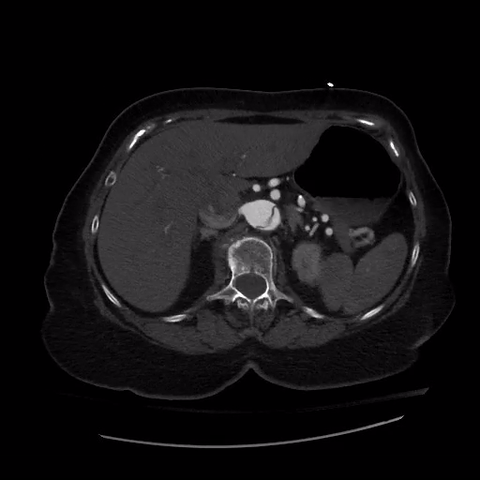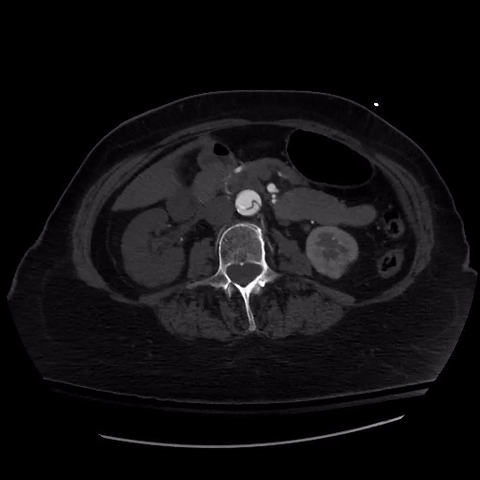
CT thorax with angiogram
TTE Parasternal Short Axis View
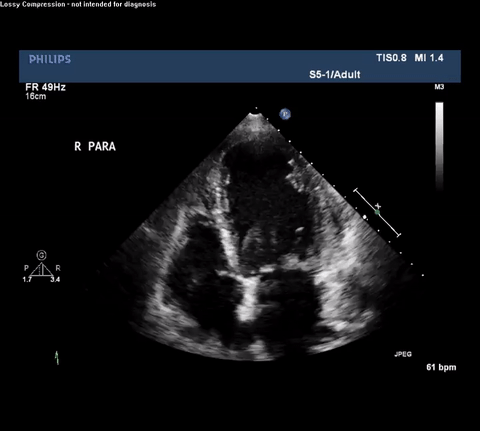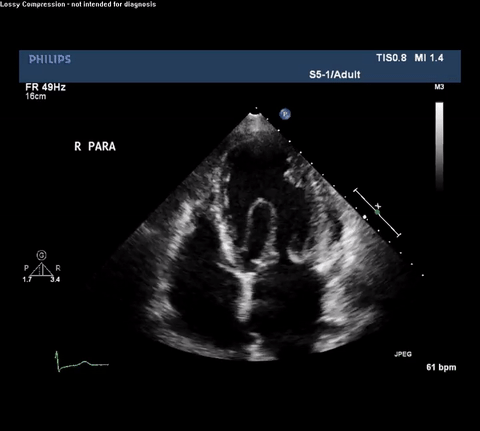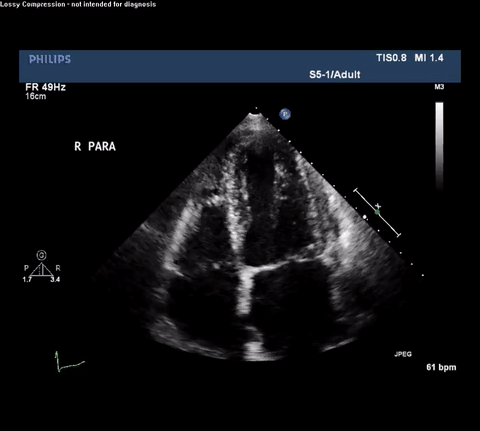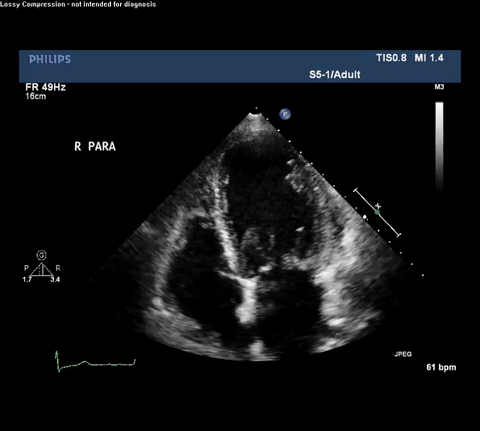
TTE Apical Four Chamber View

TTE Apical Four Chamber View With Doppler
Discussion:
In this case, POCUS resulted in the early diagnosis and prompt treatment of TA-AAD. Cardiothoracic surgery was immediately consulted, and after correcting her supratherapeutic INR, she was taken to the OR for emergent surgery. She received a bioprosthetic aortic valve and her aortic root was replaced with a synthetic graft. Her left atrial appendage was excluded with a clip to lower her long-term risk of stroke in the setting of atrial fibrillation. She had an uneventful recovery and was discharged home in stable condition.
There are two commonly described mechanisms by which TA-AAD occur. A tear in the aortic intima allows high-pressure blood to enter the media and separates the two layers. Alternatively, a rupture of the vasa vasorum allows blood to enter the aortic media creating a hematoma that forces the two layers apart. The later is considered to be much less common, accounting for only 5-10% of reported cases1.
In one large registry study, in-hospital mortality from TA-AAD was 34.9%. This study noted the highest mortality occurs in the first 7 days after the development of symptoms2. Other investigators have determined that TA-AAD has a mortality rate of 1-2% per hour over the first 24 to 48 hours3. This pattern of early mortality after the development of symptoms emphasizes the importance of making a timely diagnosis of TA-AAD.
In general, transthoracic sonography is not considered to be a reliable diagnostic tool for TA-AAD. A retrospective single center study reported on 270 patients suspected of having either an ascending aortic dissection or intramural hematoma who were initially screened for disease using transthoracic echocardiography (TTE). The reported sensitivity, specificity, positive and negative predictive values for TTE in the diagnosis of TA-AAD were 87%, 91%, 75%, and 95% respectively. The 58 patients who were correctly diagnosed by TTE experienced a rapid transit time from admission to the operating room (43 ± 25 minutes)4. However, had TTE been the sole diagnostic modality, 9 cases of TA-AAD would have been missed and these patients would have likely died. It must be further noted that this study tested TTE using a full-sized ultrasound machine, and the exams were performed and interpreted by experienced cardiologists who utilized additional views which are not routinely executed by POCUS practitioners. Thus, it is likely the test performance characteristics of cardiac POCUS for diagnosing TA-AAD are even less robust.
Despite these shortcomings, cardiac POCUS can be performed quickly and does not require either transport or IV contrast. This case illustrates how intensivists performing routine cardiac POCUS exams need to be able to identify both direct and indirect signs consistent with TA-AAD or else an opportunity to provide expedited care may be missed. For example, it is important to recognize the presence of an intimal flap which must be differentiated from reverberation or mirror artifact originating from nearby structures. Color Doppler can aid in identifying both true and false lumens along with aortic regurgitation. Finally, depending on the clinical setting other findings may be associated with TA-AAD and should raise concern for this diagnosis including: bicuspid aortic valve, dilated aortic root, aortic regurgitation and pericardial effusion.
References:
- Mann DL, Zipes DP, Libby P, Bonow RO, Braunwald E. Braunwald's heart disease : a textbook of cardiovascular medicine. Tenth edition. ed. Philadelphia, PA: Elsevier/Saunders; 2015.
- Fojtik JP, Costantino TG, Dean AJ. The diagnosis of aortic dissection by emergency medicine ultrasound. J Emerg Med. 2007;32(2):191-196.
- Chirillo F, Marchiori MC, Andriolo L, et al. Outcome of 290 patients with aortic dissection. A 12-year multicentre experience. Eur Heart J. 1990;11(4):311-319.
- Cecconi M, Chirillo F, Costantini C, et al. The role of transthoracic echocardiography in the diagnosis and management of acute type A aortic syndrome. Am Heart J. 2012;163(1):112-118.



