Reviewed By Clinical Problems Assembly
Submitted by
Tina Wang, BS
Research Associate
City of Hope National Medical Center
Duarte, CA
Kemp Kernstine, MD, PhD
Director Thoracic Oncology
City of Hope National Medical Center
Duarte, CA
Brian Tiep, MD
Director of Pulmonary Rehabilitation
City of Hope National Medical Center
Duarte, CA
K Venkataraman, MD
Director of Cardiology
City of Hope National Medical Center
Duarte, CA
David Horak, MD
Director of Pulmonary and Critical Care
City of Hope National Medical Center
Duarte, CA
Mary Barnett, RN
Clinical Coordinator Pulmonary Rehabilitation
City of Hope National Medical Center
Duarte, CA
Submit your comments to the author(s).
History
Physical Exam
Vital Signs: T: 36.8, P:82, R: 18, BP: 135/90 Well developed, well nourished male who was well oriented, HEENT: unremarkable, Chest: normally expanded with dullness to percussion in the right lower lung field with faint late inspiratory crackles and that area,
Cardiac: regular rhythm and no murmurs, S1 and S2 are normal, Abdomen: soft and non-tender with no organopathy and active bowel sounds,
Neurological: physiological, Extremities: no edema but there was early clubbing with mild cyanosis. Pulses were good.
Data
One month prior to presentation his SpO2 was 93% on room air. However, at the time of presentation his SpO2 was 80% and did not increase significantly with low flow oxygen. An arterial blood gas study performed on 100% oxygen yielded a PaO2 of 60 mm Hg, PaCO2 of 33 mm Hg, and pH of 7.33 [(A-a) pO2 gradient = 617 mm Hg] -- consistent with a right-to-left shunt of about 35%. Spirometry revealed FEV1 of 2.84L (77% of predicted), FVC of 3.87L (72% of predicted), and FEV1/FVC of 73%. His diffusion capacity was 24.9 (69% of predicted). During an oximetry walk test his SpO2 remained at 81%. An earlier incremental exercise test was terminated due to severe oxygen desaturation to 85%. Echocardiography revealed a left ventricular ejection fraction of 64%, but no evidence of intracardiac shunt. Quantitative ventilation and perfusion (V/Q) lung scan showed perfusion on the right side 42.3% and on the left side 57.7%, and ventilation on the right side 38.5%, and on the left side 61.5%, indicating some ventilation and perfusion defect.
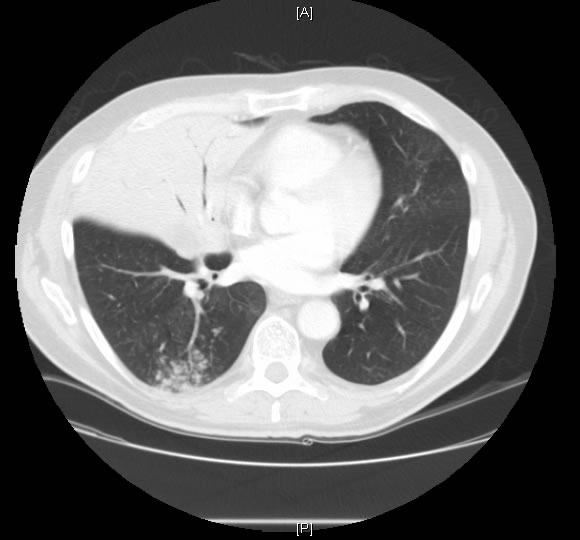
At thoracotomy the right middle lobe was completely involved with and massively distended by tumor, and there was significant hilar and intraparenchymal adenopathy. Rather than a single middle lobe vein, there were three veins, each significantly hypertrophied, 4-8mm in size. A right middle lobectomy was performed. Ligation of the middle lobe veins resulted in immediate 100% saturation from 85%. A completion right pneumonectomy was performed because of positive margins. The patient was discharged on the third post operative day without supplemental oxygen and was able to maintain his saturation above 90% while breathing room air. Pathological examination of the tumor did not reveal an anatomic cause for the intratumor shunt. There was no evidence of vascular malformation, endobronchial obstruction, or invasion of major vessels. The background lung showed evidence of emphysema. The tumor was identified as a papillary adenocarcinoma.
Discussion
This patient had an adenocarcinoma of his right lung and presented with severe refractory hypoxemia. Prior to surgery right-to-left intracardiac shunt was ruled out via echocardiography. In spite of an intrapulmonary shunt, the patient had good spirometric indices favorable for surgical resection. Preoperative suspicion that his refractory hypoxemia was due to a right-to-left intrapulmonary shunt was confirmed when saturation improved remarkably after ligation of the tumor. The tumor was identified as papillary adenocarcinoma, which shares many morphologic features with bronchioloalveolar carcinoma - growth along alveolar walls and tendency for aerogenous spread. Papillary lung carcinoma can show histopathologic features similar to those seen in the ovary or thyroid gland. Thus, a detailed clinical history is particularly advised. To the best of our knowledge, these types of tumors are no more prone to develop shunts, produce vasogenic substances or induce neoangiogenesis than other histologic types of lung adenocarcinomas. Chetty et. al3 reported a case of bronchioalveolar carcinoma in which the patient also had refractory hypoxemia that cleared upon ligation of the tumor. Pathologically, that tumor had an abundance of dilated vessels.3 We saw similar changes surgically, but not on pathological examination. An alternate explanation for our findings is that exceedingly high intralobar pressure, distal to the precapillary sphincter resulted in insufficient perfusion pressure to reach the alveoli, resulting in shunting through precapillary A-V communications and thus greater flow through the RML venous system. Previous literature on this topic made few references to intratumoral shunting. There often may be no clearly discernable anatomic/pathologic correlate for clinically obvious intrapulmonary shunting. A preoperative diagnosis may be demonstrated via V/Q scan, angiography, or pulmonary artery occlusion, but in this case the presumptive diagnosis was based on clinical suspicion and exclusion of intracardiac shunt by echocardiogram. Immediate resaturation upon removal of the tumor confirmed our suspicion that our patient was shunting through a tumor. The lesson learned from this case is that hypoxemia, instead of being a contraindication of surgery, may occasionally be correctable by surgery. Our patient easily could have been denied surgery on the basis of severe, refractory hypoxemia. Therefore pulmonary shunting through the tumor should be considered as a possible cause of refractory hypoxemia; appropriate studies should be performed to exclude this possibility before denying such patients surgery.
Figures
References
- Jian Z, Tomizawa Y, Yanagitani N, Iijima H, Sano T, Nakajima T. Papillary adenocarcinoma of the lung is a more advanced adenocarcinoma than bronchioloalveolar carcinoma that is composed of two distinct histological subtypes. Pathol Int. 2005 Oct; 55(10):619-25
- Silver SA, Askin FB. True papillary carcinoma of the lung: a distinct clinicopathologic entity. Am J Surg Pathol. 1997 Jan; 21(1):43-51
- Chetty KG, et al. Refractory hypoxemia due to intrapulmonary shunting associated with bronchioloalveolar carcinoma. Chest 1997; 111:1120-1121
- Gossage JR, Kanj G. Pulmonary arteriovenous malformations: a state of the art review. Am J Respir Crit Care Med 1998; 158:643–661
- Metin K, Karcelik M, Yavaccan O, et. al. Surgical Treatment of Pulmonary Arteriovenous Malformation: Report of Two Cases and Review of the Literature. The Journal of International Medical Research 2005; 33: 467 – 471