Reviewed By Allergy, Immunology & Inflammation Assembly
Submitted by
Lauren F. Goodman, MD
Clinical Instructor and Fellow, Pulmonary/Critical Care Medicine
The Ohio State University
Columbus, OH
Jonathan Parsons, MD, MSc
Associate Professor of Internal Medicine
Division of Pulmonary, Allergy, Critical Care and Sleep Medicine
The Ohio State University
Columbus, OH
Submit your comments to the author(s).
History
A 63-year-old woman with a history of multiple myeloma and autologous stem cell transplant 15 days prior to admission presented and was admitted with less than 1 day of fatigue, low-grade fevers and dyspnea. She denied any other symptoms, including headaches, myalgias, cough, chest pain, nausea, vomiting, constipation or diarrhea. Over the first 48 hours of her hospitalization, she developed worsening hypoxemia requiring intubation and mechanical ventilation (on post-transplant day 17).
Her myeloma was diagnosed 6 months ago and was initially treated with bortezeomib, lenalidomide and dexamethasone, as well as external beam radiation therapy to an L1 plasmacytoma 2 months prior to transplant. She underwent autologous stem cell transplantation with melphalan 200mg/body surface area approximately 2 weeks prior to admission and was discharged home 1 day prior to presentation.
Her past medical history was also positive for hypothyroidism and a history of estrogen and progesterone receptor negative breast cancer 14 years ago treated with mastectomy and 6 months of adjuvant chemotherapy. There were no records available regarding these treatments, and neither the patient nor the family were able to provide further details.
Her medications on discharge 1 day prior to readmission were prophylactic acyclovir, calcium supplement, docusate, levothyroxine, omeprazole, senna, cipro, lactulose and prochlorperazine as needed. She had a history of heavy alcohol use in the past, as well as a 40 pack-year cigarette smoking history.
Pulmonary function testing prior to transplant included spirometry, lung volumes and diffusion limit of carbon monoxide (DLCO); forced expiratory volume in 1 second (FEV1) was 1.62 liters (101% of predicted); forced vital capacity (FVC) was 1.97 liters (95% of predicted), FEV1/FVC was 82.2%. Total lung capacity was 3.39 liters (89% of predicted), and DLCO was mildly decreased at 11.5 (64% of predicted); corrected for hemoglobin it was 15.8 (87% of predicted). Resting Multi-Gated Acquisition Scan (MUGA) scan prior to transplant showed a left ventricular ejection fraction of 58% and was otherwise within normal limits.
Physical Exam
At the time of initial pulmonary/critical care consultation, she had a maximum temperature of 103 degrees Farenheit. Respiratory rate was 18 and oxygen saturation was 100% on assist control/volume control ventilation with a rate of 18, tidal volume of 450 ml (10ml/kg ideal body weight), 7.5 cmH2O positive end-expiratory pressure (PEEP) and 100% FiO2. An 8.0 oroendotracheal tube was in place. Heart was regular rhythm, mildly tachycardic, with no murmurs, rubs or gallops noted. Lungs had fair air movement bilaterally and no rales, rhonchi or wheezes.
Lab
Serum chemistry panel and liver function tests on admission were normal with the exception of magnesium of 1.5 mg/dl, potassium of 3.4 mmol/L, total protein of 4.9 g/dl and albumin of 2.3 g/dl.
Complete blood count (CBC) on admission showed a hemoglobin of 8.8 g/dl and hematocrit of 27.2% (stable compared to values from the prior hospital stay), platelet count of 93 7 K/μl (increased from 61 7 K/ μl the previous day), and a white blood cell count of 19.7 K/ μl (increased from 14 K/ μl the previous day). Differential included 3% band neutrophils, 69% segmented neutrophils, 5% lymphocytes, 4% metamyelocytes and 19% monocytes.
Urinalysis on admission showed trace bacteria with no leukocyte esterase, nitrites or white blood cells. Blood and urine cultures obtained 2 days prior to readmission were negative.
Arterial blood gas shortly prior to intubation showed a pH 7.42, Pco2 of 29 mm Hg, Po2 62 of mm Hg, bicarbonate of 23 mmol/L, and an arterial oxygen saturation of 93% on 100% FiO2.
Hospital Course
Ventilator settings were changed to a lung protective strategy, including low tidal volume ventilation at 6ml/kg ideal body weight (300ml). The patient was treated with broad-spectrum antibiotics for presumed healthcare-associated pneumonia. Tests for influenza A and B by antigen and polymerase chain reaction (PCR) were negative from nasopharyngeal swab specimens. Bronchoalveolar lavage (BAL) was performed on hospital day 3; the differential from BAL fluid included 25% alveolar macrophages, 70% neutrophils and 5% lymphocytes.
Transthoracic echocardiogram revealed normal right and left ventricular size and function, no significant valvular disease and an estimated right ventricular systolic pressure of 60mm Hg. She developed septic shock requiring pressor support; along with urine and blood cultures, BAL was repeated on hospital day 11. The differential included 80% alveolar macrophages, 13% neutrophils, 4% lymphocytes and 3% eosinophils. Comment was made of regarding “a background of prominent blood” without mention of erythrophagocytosis. Cultures from both BALs, including those for routine bacteria, acid-fast bacteria, fungi and viruses, did not grow any pathogens and BAL fluid cytology was negative. Urine and blood cultures were also repeatedly negative.
She developed atrial fibrillation with rapid ventricular response and a severe ileus. High dose steroids were added to her regimen. Despite continued low tidal volume ventilation (6ml/kg ideal body weight), she developed elevated airway pressures eventually leading to pneumothorax requiring chest tube placement. Her respiratory and hemodynamic status continued to worsen, and after discussion with family, goals of care were changed to comfort care only and she died.
Pathology/Autopsy
The right and left lungs weighed 770 gm and 590 gm, respectively (reference range: right, 480 to 680 gm; left, 420 to 600 gm), with bilateral diffuse consolidation in all lobes grossly. The pleural surface had a "cobblestone" appearance. Microscopically, bilateral lungs demonstrated diffuse alveolar septal thickening with hyaline membrane deposition. There was also focal organizing pneumonia. Diffuse squamous cell metaplasia was noted. There was no evidence of pulmonary thromboemboli in the large pulmonary arteries.
Figures

Figure 1: Posterior-anterior (PA) chest radiograph on admission showed interstitial changes and mild air space opacities which were more prominent on the right.
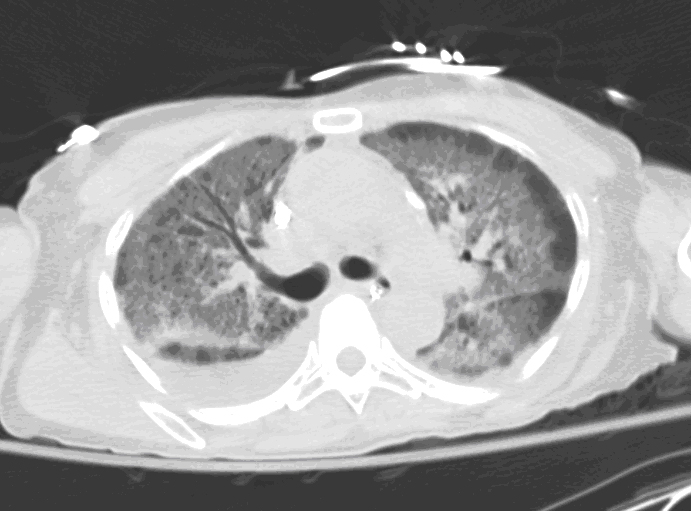
Figure 2: Axial view of high-resolution chest computed tomography (CT) using lung windows showed bilateral diffuse ground-glass infiltrates with peripheral stranding in all lobes.
References
- Limper AH. Chemotherapy-induced lung disease. Clin Chest Med 2004;25:53-64.
- Blennerhassett JB. Shock lung and diffuse alveolar damage pathological and pathogenetic considerations. Pathology 1985;17:239-247.
- Ware, LB. Pathophysiology of acute lung injury and the adult respiratory distress syndrome. Semin Respir Crit Care Med 2006;27:337-349.
- Cagle PT, Allen TC, Barrios R, Bedrossian C, Dishop MK. Color atlas and text of pulmonary pathology. 2nd ed. Philadelphia: Lippincott Williams and Wilkins; 2008.
- Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran pathologic basis of diseases. 8th ed(Professional Edition). Philadelphia: Saunders Elsevier; 2009.
- Thornburg A, Abonour R, Smith P, Knox K, Twigg HL 3rd. Hypersensitivity pneumonitis-like syndrome associated with the use of lenalidomide. Chest 2007;131:1572-1574.
- McLeod BF, Lawrence HJ, Smith DW, Vogt PJ, Gandara DR. Fatal bleomycin toxicity from a low cumulative dose in a patient with renal insufficiency. Cancer 1987;60:2617-2620.
- Keijzer A, Kuenen B. Fatal pulmonary toxicity in testis cancer with bleomycin-containing chemotherapy. J Clin Oncol 2007;25:3543-3544.
- Simpson AB, Paul J, Graham J, Kaye SB. Fatal bleomycin pulmonary toxicity in the west of Scotland 1991-95: a review of patients with germ cell tumours. Br J Cancer 1998;78:1061-1066.
- Sleijfer S. Bleomycin-induced pneumonitis. Chest 2001;120:617-624.
- Uzel I, Ozguroglu M, Uzel B, Kaynak K, Demirhan O, Akman C, Oz F, Yaman M. Delayed onset bleomycin-induced pneumonitis. Urology 2005;66:195.
- Segura A, Yuste A, Cercos A, López-Tendero P, Gironés R, Pérez-Fidalgo JA, Herranz C. Pulmonary fibrosis induced by cyclophosphamide. Ann Pharmacother 2001;35:894-897.
- Hamada K, Nagai S, Kitaichi M, Jin G, Shigematsu M, Nagao T, Sato A, Mishima M. Cyclophosphamide-induced late-onset lung disease. Intern Med 2003;42:82-87.
- Brieva J. Cyclophosphamide-induced acute respiratory distress syndrome. Respirology 2007;12:769-773.
- Adam A, Dixon AK. Grainger & Allison’s diagnostic radiology: a textbook of medical imaging. 5th ed. Philadelphia: Churchill Livingstone; 2008.
- Miyakoshi S, Kami M, Yuji K, Matsumura T, Takatoku M, Sasaki M, Narimatsu H, Fujii T, Kawabata M, Taniguchi S, Ozawa K, Oshimi K. Severe pulmonary complications in Japanese patients after bortezomib treatment for refractory multiple myeloma. Blood 2006; 107: 3492-3494.
- Ohri A, Arena FP. Severe pulmonary complications in African-American patient after bortezomib therapy. Am J Ther 2006; 13:553-555.
- Cañete C, Soley A, Vuelta Arce M, Escoda L. Pulmonary toxicity after bortezomib. Farm Hosp 2008; 32:301-302. [Article in Spanish]
- Erasmus JJ, McAdams HP, Rossi SE. Drug-induced Lung Injury. Semin in Roentgen 2002;37:72-81.



