Reviewed By Critical Care Assembly
Submitted by
Jing Wang, PhD, PAC
Department of Neurosurgery, Department of Medicine, Critical Care
Inova Fairfax Hospital
Falls Church, VA
Nilesh Vyas, MD
Department of Neurosurgery
Inova Fairfax Hospital
Falls Church, VA
Laith Altaweel, MD
Department of Medicine, Critical Care
Inova Fairfax Hospital
Falls Church, VA
Submit your comments to the author(s).
History
Case Presentation
This is a 21-year-old right-handed male, with a past medical history significant for asthma and Attention Deficit Disorder (ADD), treated with methylphenidate, who was found in a gym bathroom with left sided weakness and urinary incontinence shortly after lifting weights. Upon arrival to the emergency department (ED), he was following commands with left hemiparesis. He complained of severe headache. Initial laboratory studies revealed a normal platelet count, coagulation profile, and negative toxicology screen.
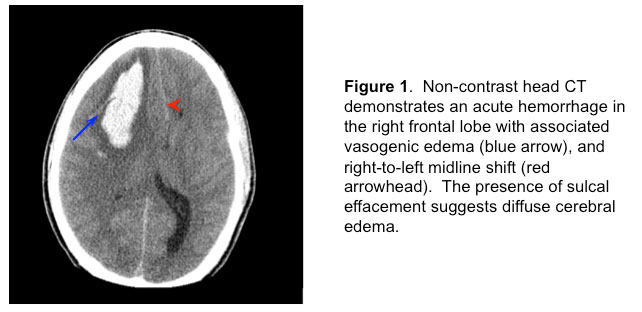
Emergent non-contrast head CT revealed a right frontal intracranial hemorrhage (Figure 1)
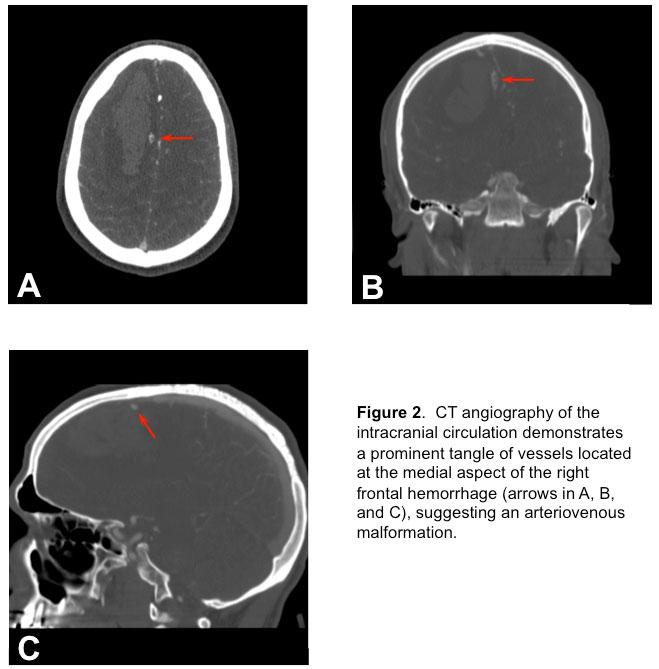
and a CT angiography (CTA) of the brain (Figure 2)
revealed an arterio-venous malformation (AVM). Soon after presentation, he became more lethargic with episodes of bradycardia to the 30s and without concomitant hypertension. He was subsequently intubated for airway protection with propofol and rocuronium. In addition, 50 grams of mannitol was administered intravenously due to concerns of rising intracranial pressure (ICP). Now intubated, propofol infusion was titrated to maintain deep sedation (no response to painful stimuli). He was then moved to the neurointensive care unit (NSICU).
Social history is notable for smoking cigarettes. There is no family history of vascular malformations or subarachnoid hemorrhage.
Physical Exam
Initial Physical Exam
The patient was examined in the ED prior to intubation. His head of bed was at 30 degrees. Vitals signs were as follows: temperature of 95.5 °F, blood pressure (BP) of 130/79 mmHg, pulse of 63, respiratory rate of 20, and oxygen saturation of 98% on 3 liters/minute nasal cannula. He was diaphoretic. His lungs were clear to auscultation bilaterally. He had a regular heart rate with normal S1 and S2. No appreciable murmurs, rubs or gallops. His abdomen was soft, nontender, and nondistended with positive bowel sounds. He has no lower extremity edema. He had 2+ dorsalis pedis pulses. Neurologically, his Glasgow coma scale (GCS) was 15 (Table 1). He opened eyes to voice (E4); he was oriented to person, place and date (V5); and he had a dense left hemiparesis but was able to move his right side against gravity to commands (M6). His speech was fluent. He had right gaze preference. His pupils were 4 mm, equal in size and equally reactive to light bilaterally. He had a left facial droop. Babinski’s sign was absent.
Lab
Laboratory and Imaging Findings
Laboratory Findings:
| Test (Abbreviation) |
Value (normal unless otherwise indicated) |
|
White Blood Cell count (WBC) |
11.8 x 103/µL |
|
Hemoglobin (Hb) |
12.1 g/dL |
|
Hematocrit (Hct) |
35.7% |
|
Platelets |
346 x 103/µL |
|
Prothrombin Time (PT) |
14.5 sec |
|
Partial Thromboplastin Time (PTT) |
27 sec |
|
International Normalized Ratio (INR) |
1.1 |
|
Blood Urea Nitrogen (BUN) |
24 mg/dL |
|
Na+ |
136 mEq/L |
|
K+ |
3.8 mEq/L |
|
CO2 |
23 mEq/L |
|
Creatinine |
1.5 mg/dL |
|
Glucose |
117 mg/dL |
Urine toxicology, including alcohol, amphetamines, barbiturates, benzodiazepines, cocaine, heroin, Phencyclidine (PCP), and Tetrahydrocannabinol (THC), were all negative.
Imaging Findings:
1) Brain non-contrast CT (Figure 1) showed a large right frontal intraparenchymal hemorrhage with 7-mm midline shift and partial effacement of the basilar cisterns.
2) Brain CTA showed the appearance of an apparent AVM supplied by the anterior cerebral arteries with superficial drainage at the superior sagittal sinus.
3) Chest x-ray was clear.
Hospital Course / Case Resolution
Over a period of hours, while in the NSICU, the patients GCS declined from 15 to 8. Given his deteriorating neurological status, a parenchymal fiber optic bolt was placed to measure his ICP. His initial ICP was 30 mmHg (normal < 20 mmHg), for which he received rapid intravenous boluses of 50 grams of mannitol and 500 mL of 3% saline. Thereafter, 250 mL of intravenous 3% saline was administered every 6 hours to maintain a serum sodium range of 145-150 mEq/L. A radial arterial catheter was placed to maintain a cerebral perfusion pressure (CPP = Mean Arterial Pressure - Intracranial Pressure) between 60 and 80 mmHg with norepinephrine intravenous drip titration. Sedation with propofol and fetanyl was no longer held once ICP monitoring was initiated. Prophylactic antiepileptic treatment was initiated with 1.5 grams of phenytoin (20 mg/kg loading) to obtain a therapeutic level (10-20 total level or 1-2 free) and maintained with 100 mg IV infusion every 8 hours. In order to monitor for nonconvulsive seizures, continuous electroencephalogram (EEG) monitoring was used.
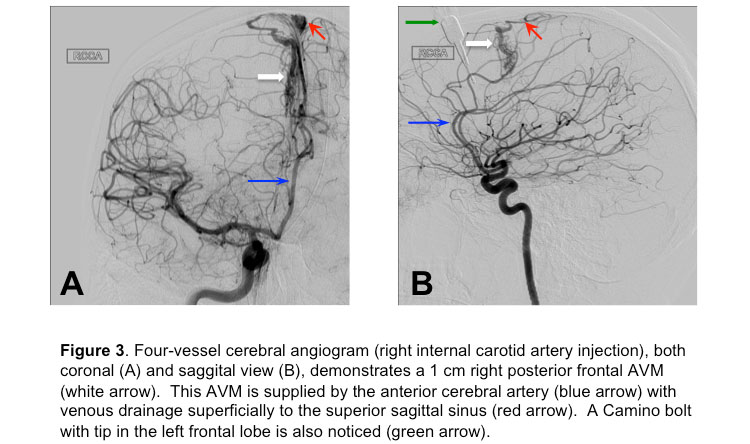
After the initial few hours of stabilization, the patient was moved to the angiography suite for a 4-vessel angiogram, which confirmed an AVM in the right frontal lobe (Figure 3),
with no associated aneurysms.
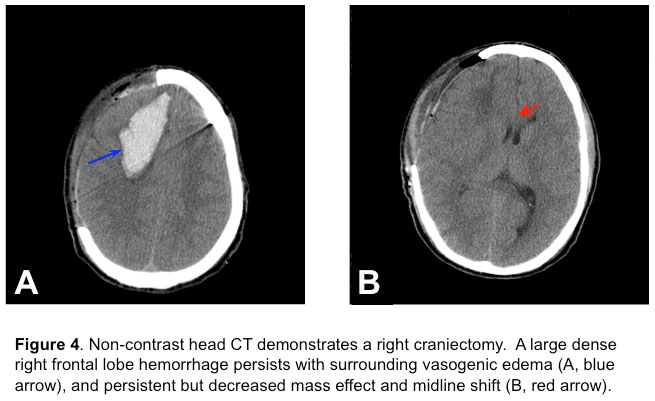
Over the subsequent 24 hours, the patient’s ICP climbed up to 40 mmHg, despite multiple boluses of 3% saline (Na+ climbed to 147 mEq/dL from 136 mEq/dL on admission) and mannitol, heavy sedation, paralysis and active cutaneous cooling (Arctic Sun) to maintain normothermia. Due to refractory ICP elevation, the patient underwent an emergent right fronto-temporo-parietal decompressive craniectomy (Figure 4)
without removal of the hematoma or AVM. The ICP after the decompressive hemicraniectomy was less than 20 mmHg.
On postoperative day 0 and post-bleed day 1, the patient followed simple commands by showing 2 fingers on the right side. On postoperative day 1, the patient developed septic shock with fever of 103.6 °F, fluid refractory hypotension, oliguria, increasing serum lactate and leukocytosis. Escalating doses of norepinephrine infusion were necessary to maintain the CPP goal. A new left lower lobe infiltrate on chest xray was noted and cultures were sent from blood, urine, and sputum. Empiric antibiotic coverage was initiated with vancomycin and doripenem, and de-escalated within 48 hrs to vancomycin alone when sputum culture grew Methicillin-Resistant Staphylococcus Aureus (MRSA), and overall clinical improvement (defervescence and hemodynamic stability). All other cultures and surgical incision site did not suggest additional sources of infection. Induced normothermia was initiated during this period, with reductions in water temperature used as a surrogate for fever. During this period, a mild elevation in INR (1.5) was corrected with transfusion of fresh frozen plasma.
The patient was started on sequential compression devices for deep vein thrombosis prophylaxis on admission and subcutaneous heparin on postoperative day 3. On postoperative day 5, the patient was awake, alert, and oriented to person, place and date. He had fluent speech with a mild dysarthria and left facial droop. His sensation was intact and motor strength were intact except for persistent right sided weakness. His motor exam improved on the left side: upper extremity strength still flaccid 0/5 but the lower extremity improved from flaccid to hip flexion 2/5, left knee extension 5-/5, and left foot dorsiflexion 4-/5 (Table 2). Right upper and lower limb strength were normal, as had been on admission. His sensation was intact to light touch. He passed a swallow evaluation and was started on a mechanical soft diet. He continued to participate with physical and occupational therapy. He was transferred to the floor on postoperative day 7 and was later discharged to an acute rehabilitation center.
One month after hospital discharge, he was readmitted for an elective right frontal craniotomy with AVM resection and evacuation of old intraparenchymal hemorrhage and large cranioplasty. He was discharged 2 days later. By his 4-month follow up visit, he has resumed most of his regular activities with only minor weakness in his left hand.
References
- Qureshi A, Tuhrim S, Broderick J, et al. Spontaneous intracerebral hemorrhage. N Engl J Med 2001;344:1450–1460.
- Wong GKC, Siu DYW, Abrigo JM, et al. Computed tomographic angiography and venography for young or nonhypertensive patients with acute spontaneous intracerebral hemorrhage. Stroke 2011;42:211–213.
- Zhu XL, Chan MS, Poon WS. Spontaneous intracranial hemorrhage: which patients need diagnostic cerebral angiography? A prospective study of 206 cases and review of the literature. Stroke 1997;28:1406–1409.
- Cordonnier C, Klijn CJM, van Beijnum J, et al. Radiological investigation of spontaneous intracerebral hemorrhage: systematic review and trinational survey. Stroke 2010;41:685–690.
- Park J, Hwang Y-H, Baik SK, et al. Angiographic examination of spontaneous putaminal hemorrhage. Cerebrovasc Dis 2007;24:434–438.
- Hino A, Fujimoto M, Yamaki T, et al. Value of repeat angiography in patients with spontaneous subcortical hemorrhage. Stroke 1998;29:2517–2521.
- Buis DR, Van Den Berg R, Lagerwaard FJ, et al. Brain arteriovenous malformations: from diagnosis to treatment. J Neurosurg Sci 2011;55:39–56.
- Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg 1986;65:476–483.
- Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg 2003;98:3–7.
- Morgenstern LB, Hemphill JC, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41:2108–2129.
- Venkatasubramanian C, Mlynash M, Finley-Caulfield A, et al. Natural history of perihematomal edema after intracerebral hemorrhage measured by serial magnetic resonance imaging. Stroke 2011;42:73–80.
- Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet 2005;365:387–397.
- Zazulia AR, Diringer MN, Derdeyn CP, et al. Progression of mass effect after intracerebral hemorrhage. Stroke 1999;30:1167–1173.
- Kontos HA, Wei EP, Raper AJ, et al. Local mechanism of CO2 action of cat pial arterioles. Stroke 1977;8:226–229.
- Koenig MA, Bryan M, Lewin JL, et al. Reversal of transtentorial herniation with hypertonic saline. Neurology 2008;70:1023–1029.
- Cruz J, Minoja G, Okuchi K, et al. Successful use of the new high-dose mannitol treatment in patients with Glasgow Coma Scale scores of 3 and bilateral abnormal pupillary widening: a randomized trial. J Neurosurg 2004;100:376–383.
- Mortazavi MM, Romeo AK, Deep A, et al. Hypertonic saline for treating raised intracranial pressure: literature review with meta-analysis. J Neurosurg 2012;116:210–221.
- Schwarz S, Schwab S, Bertram M, et al. Effects of hypertonic saline hydroxyethyl starch solution and mannitol in patients with increased intracranial pressure after stroke. Stroke 1998;29:1550–1555.
- Ichai C, Armando G, Orban J-C, et al. Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intensive Care Med 2009;35:471–479.
- Pérez-Bárcena J, Llompart-Pou JA, Homar J, et al. Pentobarbital versus thiopental in the treatment of refractory intracranial hypertension in patients with traumatic brain injury: a randomized controlled trial. Crit Care 2008;12:R112.
- Claassen J, Hirsch LJ, Emerson RG, et al. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 2002;43:146–153.
- Clifton GL, Miller ER, Choi SC, et al. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med 2001;344:556–563.
- Sinclair HL, Andrews PJ. Bench-to-bedside review: Hypothermia in traumatic brain injury. Crit Care 2010;14:204.
- Cooper DJ, Rosenfeld JV, Murray L, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med 2011;364:1493–1502.